Mix and match
advertisement

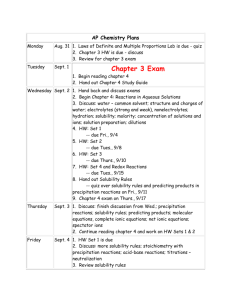
Mix and match PRECIPITATION LAB Objective Predict double replacement reactions, observe them, and express them as using chemical equations, total ionic equations, and net equations. Make a table to list these, write the chemical formulas, and briefly describe each solution • A list down – A1 Copper (II) nitrate – A2 Nickel (II) nitrate – A3 Cobalt (II) nitrate • B list across – B1 Sodium carbonate – B2 Sodium chloride A1: Name Formula Description A2 Name Formula Description A3 Name Formula Description B1: B2: Name Formula Description Name Formula Description EXAMPLE A1 • Copper (II) nitrate • Cu(NO3)2 • Blue clear liquid Predict products and predict if the reaction between A and B happens • Predict based on double replacement • Write formula of each product • Check on the solubility chart and note • If both dissolve, no reaction • If there is a precipitate, reaction A1: Name Formula Description A2 Name Formula Description A3 Name Formula Description B1: B2: Name Formula Description Name Formula Description Predict products Note solubility Predict Predict products Note solubility Predict Predict products Note solubility Predict Predict products Note solubility Predict Predict products Note solubility Predict Predict products Note solubility Predict Example block at intersection of A1 and B1 • CuCO3 I • NaNO3 S • Predict reaction? + or - Mix • In spot plate, set up reactions between A and B chemicals, 6 reactions in all • Combine 3 drops of each in wells of spot plate and record observations A1: Name Formula Description A2 Name Formula Description A3 Name Formula Description B1: B2: Name Formula Description Name Formula Description Predict products Note solubility Predict products Note solubility Mix and observe Mix and observe Predict products Note solubility Predict products Note solubility Mix and observe Mix and observe Predict products Note solubility Predict products Note solubility Mix and observe Mix and observe Example block at intersection of A1 and B1 • CuCO3 I • NaNO3 S • Predict reaction? + or • Describe what you actually see when you mix 3 drops of each chemical in the well between them Write • For TWO reactions: – Write chemical equation – Write total ionic equation – Write net ionic equation • For TWO non-reactions – Write chemical equation – Write total ionic equation – Explain why the reaction did not happen using the numbered solubility rules