carbohydrates - UniMAP Portal
advertisement

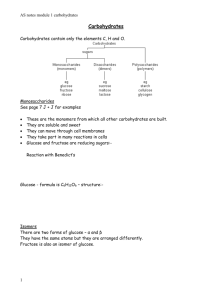
CARBOHYDRATES Carbohydrates are a major energy source for living organisms Carbohydrates always have a 1:2:1 ratio of carbon, hydrogen, and oxygen. Mitochondria break down carbohydrates and use the chemical energy released from its bonds for its live activities CLASSFICATION OF CARBOHYDRATES MONOSACCHARIDES – simple sugars classified according to the number of carbon atoms they contain: 3 carbons: trioses (glyceraldehyde) 4 C: tetroses (erythrose) 5 C: pentoses (ribose) 6 C: hexoses (glucose) 7 C: heptoses (sedoheptulose) 9 C: nonoses (neuraminic acid) ALDOSES AND KETOSES Carbohydrates with an aldehyde (-CHO) as their most oxidized functional group are called aldoses e.g. glyceraldehyde (CH2OH-CHOH-CHO); Those with a keto group (-C=O) as their most oxidized functional group are ketoses e.g.dihydroxyacetone (CH2OH-C=O-CH2OH) Aldose carbohydrates normally have a suffix “-ose” and ketoses end with suffix “ulose”. GLYCOSIDES • Monosaccharides can be linked by glycosidic bonds (joining of 2 hydroxyl groups of sugars by splitting out water molecule) to create larger structures. • Disaccharides contain 2 monosaccharides e.g. lactose (galactose+glucose); maltose (glucose+glucose); sucrose (glucose+fructose) • Oligosaccharides – 3 to 12 monosaccharides units e.g. glycoproteins • Polysaccharides – more than 12 monosaccharides units e.g. glycogen (homopolysaccharide) having hundreds of sugar units; glycosaminoglycans (heteropolysaccharides) containing a number of different monosaccharides species. SIMPLE SUGARS • • • • The monomers of carbohydrates are called monosaccharides and are also called simple sugars. They are usually ring-like and are composed of five or six carbons. They are either a polyhydroxy aldehyde or a polyhydroxy ketone, which means they have more than one hydroxide group (-OH) and one carbonyl group (C=O). Some popular monosaccharides are glucose, fructose, and galactose STRUCTURES OF SUGARS ISOMERS- compounds that have the same chemical formula e.g. fructose, glucose, mannose, and galactose are isomers of each other having formula C6H12O6. EPIMERS- refer to sugars whose configuration differ around one specific carbon atom e.g. glucose and galactose are C-4 epimers and glucose and mannose are C-2 epimers. ENANTIOMERS- a special type of isomerism found in pairs of structures that are mirror images of each other. The mirror images are termed as enantiomers and the two members are designated as D- and L- sugar. The vast majority of sugars in humans are D-sugars. CYCLIZATION OF SUGARS- most monosaccharides with 5 or more carbon atoms are predominately found in a ring form, where the aldehyde or ketone group has reacted with an alcoholic group on the same sugar group to form a hemiacetal or hemiketal ring. Pyranose ring- if the ring has 5 carbons and 1 oxygen. Furanose ring- if the ring is 5-membered (4 carbons and 1 oxygen). STRUCTURES Cotnd. ANOMERIC CARBON- formation of hemiacetal or hemiketal ring results in the creation of an anomeric carbon at carbon 1 of aldose and carbon 2 of a ketose. These structures are termed as α or β configurations of the sugar e.g. α-D-glucose (C5 H10 O5 - H-C-OH) or β-D- glucose (C5 H10 O5 - OH-C-H). Enzymes are able to distinguish between these two structures and use one the other preferentially. STRUCTURES Cotnd. MUTAROTATION- the cyclic α and β anomers of a sugar in solution are in equilibrium with each other, and can readily be interconverted. This process requires the ring structure open to linear form during the interconversion and is resulted due to the characteristic changes in the optical rotation of polarized light. REPRESENTATION OF SUGAR CONFORMATION. The hemiacetal ring of sugar is called a ‘Fisher projection’. It also can be represented by the cyclic form of ‘Haworth projection’. A 3-dimensional conformation of sugars in nature can be represented in a ‘boat’ and ‘chair forms. D- and L- enantiomers FISHER PROJECTION GLUCOSE - HAWORTH PROJECTION FRUCTOSE AND RIBOSE REACTIONS INVOLVING MONOSACCHARIDES Reducing sugars- if the oxygen on the anomeric carbon (the carbonyl group) of a sugar is not attached to any other structure, that sugar is a reducing sugar. A reducing sugar can react with chemical reagents (e.g. Benedict’s solution) and reduce the reactive component. The anomeric carbon itself becomes oxidized. Oxidation-reduction reactions- oxidation of the –CH2-OH group at the carbon 6 produces a – uronic acid. Reduction of the carbonyl carbon (aldehyde or keto group) produces a new alcohol group (polyols) e.g. glucose is reduced to sorbitol, fructose to sorbitol and mannitol, galactose to galactitol. Reduction of hydroxyl groups produce deoxysugars e.g. ribose is converted to 2-deoxyribose, an essential component of DNA. Reactions cotnd. Carbohydrates can also be attached by glycosidic bonds to non-carbohydrate structures to form ‘complex carbohydrates’. The non-carbohydrate portion of the molecule is called the aglycone and the entire molecule can be called by the generic name glycoside. The aldose, the carbon 1 or ketose, the carbon 2, which participates in the glycosidic link is called the a glycosyl residue. E.g. if the anomeric carbon of glucose participates in such a bond, that sugar is called a glucosyl residue. If the identity of the attached sugar is known, its name can be used – glucoside or galatoside. OLIGOSACCHARIDES • • These contain 2-10 units of monosaccharides (monomers) linked to each other via glycosidic linkages, e.g. sucrose, lactose, maltose, raffinose, stachyose etc. Glycosidic bonds: The anomeric hydroxyl group and a hydroxyl group of another sugar or some other compound can join together, splitting out water to form a glycosidic bond. Glycosidic bond diagram Glucosidic bond cont. SUCROSE LACTOSE MALTOSE POLYSACCHARIDES Carbohydrates which are composed of more than then monosaccharide units linked to each other through glycosidic bonds are referred to as polysaccharides. These include carbohydrates like cellulose, starch, glycogen, fructans, arabinans etc. CELLULOSE GLYCOGEN