Introduction to Matter
advertisement

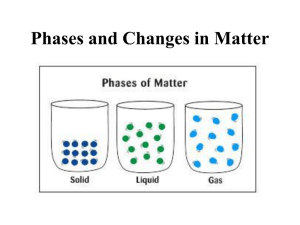
Matter 1. Matter-Anything that takes up space and has mass. Mass is the measure of the amount of matter an object contains. A balance measures mass Weight is a measure of the pull of gravity on the matter in an object; *spring scales measure weight (pull of gravity) **Weight can change but mass does not 2. 3. Volume-how much space an object occupies Regular objects (definite shapes): V=lwh *a box, table, ice cube, etc. Irregular shapes: displacement of water *beans in a graduated cykinder 1. Atoms are extremely small. it would take a stack of about 50,000 aluminum atoms to equal the thickness of a sheet of aluminum foil from your kitchen. if you could enlarge a penny until it was as wide as the US, each of its atoms would be only about 3 cm in diameter – about the size of a ping-pong ball a human hair is about 1 million carbon atoms wide. a typical human cell contains roughly 1 trillion atoms. a speck of dust might contain 3x1012 (3 trillion) atoms. it would take you around 500 years to count the number of atoms in a grain of salt. www.deckersfoods.com C-C-C-C-C-… + 999,995 more 1 trillion atoms . Is made of approximately 3 trillion atoms Just one of these grains 2. Molecule-2 or more atoms combined ozone This can be the same kind of atoms or different atoms. 3. Atoms and molecules are always in motion water 1. Particle arrangement and energy determines the state a. Solid-have a definite volume and definite shape; molecules vibrate in place-low kinetic energy b. Liquid-No definite shape but definite volume; molecules move slowly (kinetic energy increases) c. Gas-no definite shape or volume; move very fast with high kinetic energy Close up view of atoms and their behavior Animated images are from http://www.chem.purdue.edu/gchelp/ What states of matter are represented in the photograph? Kinetic energy increases as heat is added YOU MAY WANT TO DRAW THIS d. Plasma-a special state •Makes up 99% of the visible universe •The most common form of matter •A charged gas full of energy •Plasma can be found in the Sun and … Stars and … Lightning a. Changing states requires a change in pressure or temperature b. Processes of change 1. gas to liquid condensation 2. liquid to solid freezing 3. solid to liquid melting 4. Liquid to gas evaporation, boiling Materials differ in terms of the kind of matter they contain. Matter that has a uniform and definite composition is called a substance. Substances can be identified as either an element, compound, or a mixture. • A pure substance that cannot be broken down any further • contain just one type of atom • Atoms are the smallest part of an element • Can exist as a single atom or a molecule (2 atoms joined) • Symbol-represents an element • Examples might be Oxygen (O), Nitrogen (N), Carbon (C) and Hydrogen (H). • These 4 are necessary to all life contains two or more different atoms joined together. This is a molecule and it is the smallest part of a compound a chemical reaction is needed to separate elements in a compound. Examples would be water, salt, sugar Represented by formulas: H2O, NaCl, C6H12O6 3. mixture 1. A mixture contains two or more different substances that are only be physically joined together, not chemically. A mixture can contain both elements and compounds. There are two kinds of mixtures. a. Homogenous -equal parts; evenly mixed solutions like saltwater or koolaid. b. Homogeneous -uneven mix of parts. Many settle out depending on weight. Examples might include milk, muddy water and salad dressing E. Properties of Matter 1. Properties used to describe matter can be classified as: a. Extensive – depends on the amount of matter in the sample b. - Mass, volume, calories are examples Intensive – depends on the type of matter, not the amount present - Hardness, Density, Boiling Point 2. Types of properties are… a. Physical Properties- a property that can be observed and measured without changing the material’s composition (identity). -Examples- color, hardness, melting point, boiling point, texture, odor, size b. Chemical Properties- a property that can only be observed by changing the composition (identity) of the material. -Examples- ability to burn, decompose, ferment, react with oxygen, etc. 1. Physical change- A type of change that alters a material without changing it’s chemical composition. Boil, melt, cut, bend, split, crack Is boiled water still water? Is a cut piece of wood still wood? Can be reversible, or irreversible 2. Chemical change - a change where a new substance is formed that is different than the original Examples: Burning, corroding, decomposing Evidence of a chemical change include Energy (light, heat or both) is absorbed or released Endothermic-heat is absorbed cooling temperatures Exothermic-heat is released raising temperatures and often giving off light Color changes Gas production (bubbling, fizzing, or odor change; smoke)