Chapter 6 The Periodic Table and Periodic Law
advertisement
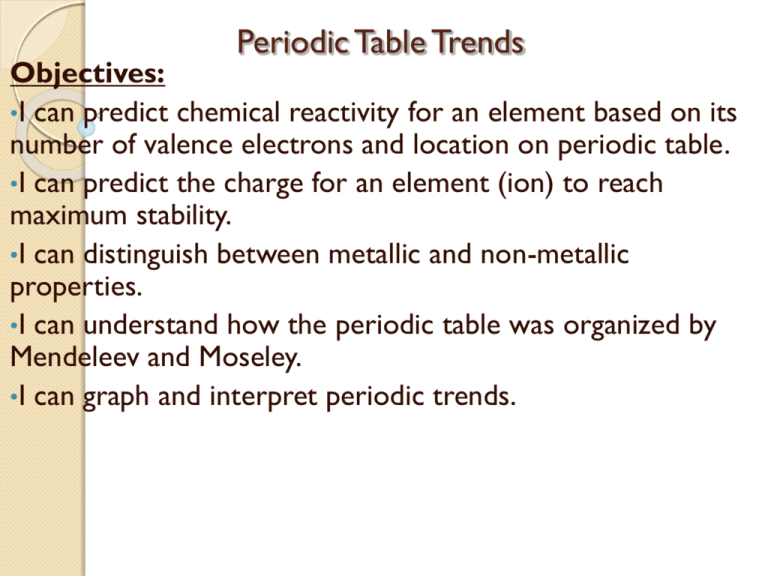
Periodic Table Trends Objectives: •I can predict chemical reactivity for an element based on its number of valence electrons and location on periodic table. •I can predict the charge for an element (ion) to reach maximum stability. •I can distinguish between metallic and non-metallic properties. •I can understand how the periodic table was organized by Mendeleev and Moseley. •I can graph and interpret periodic trends. Chemistry 1/8/14 Objectives: Review Classroom Expectations Analyze first semester grades. Review Chemical Stability Concepts Chemistry Midterm make-ups addressed ASAP Homework: Classroom Expectation Hand-out signed/returned. Complete Chemical Stability Worksheet ICP: /8/13 Objectives: Review Classroom Expectations Analyze first semester grades. Motion on Graph Worksheet Chemistry Midterm make-ups addressed ASAP Homework: Student/Parent sign Classroom Expecation Hand-out Complete Motion on Graph Worksheet Chemistry 1/10/14 Objectives: Analyze first semester grades. Review Periodic Trends Graphs Review Chemical Stability Concepts Homework: Classroom Expectation Hand-out signed/returned. Complete Chemical Stability Worksheet Analyze First Semester Grades Address reasons for scores. Address your graphs Periodic Properties and Trends Atomic Radius: -Size of an atom. -The distance from the nucleus to the outermost energy level in picometers, pm. (1pm = 1x10-12 m) Ionization Energy: -The amount of energy needed to remove a valence electron from an atom. -The amount of energy needed to overcome the attractive force the ve- has with protons in the nucleus. -Energy required for an atom to become a cation, more stable. Electronegativity: -The degree of attraction one atom’s protons has toward another atom’s ve-. -Determines the type of bond between the atoms, ionic or covalent. Chemistry 1/13/14 Due: Chemical Stability Cross Word Puzzle Classroom Expectation Hand-Out signed. Objectives: Review Chemical Stability Concepts Review Periodic Trends Graphs Homework: Periodic Table Worksheet Chemical Stability Worksheet Periodic Trends: Atomic Radius Across a Period •Decreases Atomic Number vs. Atomic Radius 200 atomic raidus (pm) 180 160 140 Down a Group •Increases 120 100 80 60 40 20 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 atomic number Periodic Table : Atomic Radius Periodic Trends: Ionization Energy Atomic Number vs. Ionization Energy 2500 Ionization Energy (kJ/mol) Across a Period: •Increases 2000 1500 1000 Down a Group: •Decreases 500 0 1 3 5 7 9 11 Atomic Number 13 15 17 Periodic Trend: Electronegativity Across a Period: •Increases except noble gases. Down a Group: •Decreases except for noble gases. mmsphyschem.com Electronegativity and Chemical Bonding •Do metals or non-metals have a greater electronegativity value? Periodic Trends Analysis To determine why your term(atomic radius, ionization energy, or electronegativity) exhibits this trend across a period and down a group. Think about the definition of your term and how it is affected across a period and down a group. Periodic Trend Values 1. Use your atomic radius graph to rank the following elements in increasing atomic radius. Cl, Mg, Al, Na 2. Use your ionization energy graph to rank the following elements in decreasing ionization energy. Ba, Mg, Ca, Be Chemical Stability Octet Rule: Atoms will gain, lose, or share valence electrons to reach maximum stability. What is maximum stability for most atoms? 8 valence electrons (ve-) Exceptions: H and He max. stability = 2 ve- How do atoms achieve stability ? Atoms chemically bonding with other atoms. Formation of diverse compounds in nature. Chemical Stability: Octet Rule Metals will lose ve- to reach stability. Form a cation (+ charged) www.teacherfurse.com Non-metals will gain ve- to reach stability. Form an anion (- charged) http://www.green-planet-solar-energy.com/the-element-chlorine.html Chemical Stability-Key Determine what type of charge each element below would form to reach maximum stability. a. b. c. d. e. Sodium Na1+ Oxygen O2Argon Ar Phosphorus p3Chromium Cr2+ Size of an Ion Use the diagram below to determine what happens to the size of a neutral atom(parent atom) when it becomes an ion. Size of a Cation Why is the cation smaller than its parent atom (neutral)? Size of a Cation A cation is smaller than its parent atom. Why? Because metals will lose an energy level in the process of becoming a cation. Size of an Anion Why is an anion larger than its parent atom? Size of an Anion A anion is larger than its parent isotope. Why? Repulsion force increases as more electrons are added to the outer most energy level. Swells the energy leve Size of Ions a. Circle the atom that is larger in size. Ca or Ca2+ b. S or S2- 1. Circle the atom that is smaller in size. a. Al or Al3+ 2. b. N or N 3-





