Chemical Reactions
advertisement

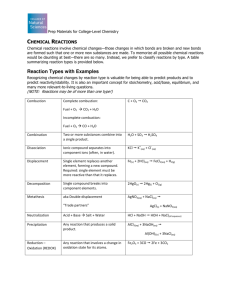
Chemical Reactions Types of Reactions • There are five types of chemical reactions we will talk about: 1. 2. 3. 4. 5. • Synthesis reactions Decomposition reactions Single displacement reactions Double replacement reactions Combustion reactions You need to be able to identify the type of reaction and predict the products Steps to Writing Reactions • Some steps for doing reactions 1. 2. 3. Identify the type of reaction Predict the products using the type of reaction as a model Balance the equation Don’t forget about the diatomic elements! In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! 1. Synthesis reactions • • Synthesis reactions occur when two substances (generally elements) combine and form a compound. reactant + reactant 1 product Basically: A + B AB • • Example: 2H2 + O2 2H2O Example: C + O2 CO2 The key to recognizing a synthesis reaction is to look for only one product! Synthesis Reactions Practice Predict the products. Write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas • Magnesium reacts with fluorine gas • Aluminum reacts with oxygen gas 2. Decomposition Reactions • Decomposition reactions occur when a compound breaks up into the elements or to simpler compounds 1 Reactant Product + Product • In general: AB A + B • Example: 2 H2O 2H2 + O2 • The key to recognizing decomposition reactions is to look for only one reactant. Decomposition Reactions Practice • Predict the products. Then, write and balance the following decomposition reaction equations: Solid Lead (IV) oxide decomposes • Solid Aluminum nitride decomposes • Iron (III) chloride decomposes • Practice Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: Nitrogen and oxygen react to form nitrogen monoxide. Solid cobalt reacts with solid sulfur to form cobalt (III)sulfide. Solid nitrogen triiodide decomposes. Single replacement reactions She’s seeing another man! 3. Single Replacement Reactions • • • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal OR a nonmetal can replace a nonmetal . element + compound new element + new compound A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) Key to recognizing single replacement reactions: Look for a pure element on each side of the equation. K + H20 KOH + H2 Single Replacement Reactions Single Replacement Reactions Write and balance the following single replacement reaction equations: • Zinc metal reacts with aqueous hydrochloric acid (HCl) • Bromine gas reacts with sodium iodide. • Silver reacts with aqueous potassium nitrate. Single Replacement Reactions More practice • • Sodium chloride solid reacts with fluorine gas Aluminum metal reacts with aqueous copper (II) nitrate 4. Double Replacement Reactions • • Double Replacement Reactions occur when a metal replaces a metal in a compound and a nonmetal replaces a nonmetal in a compound Compound + compound new compound + new compound • AB + CD AD + CB Double replacement reactions: • Examples AgNO3 + NaCl AgCl + NaNO3 K2SO4 + Ba(NO3)2 KNO3 + BaSO4 The ions switch partners, like in square dancing. Practice Predict the products. Balance the equation. HCl(aq) + AgNO3(aq) CaCl2(aq) + Na3PO4(aq) Pb(NO3)2(aq) + BaCl2(aq) FeCl3(aq) + NaOH(aq) H2SO4(aq) + NaOH(aq) 5. Combustion Reactions • • • • Combustion reactions occur when a substance reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel 2) Oxygen to burn it with 3) Something to ignite the reaction (spark) Combustion Reactions • • • In general: CxHy + O2 CO2 + H2O Products in combustion of hydrocarbons are ALWAYS carbon dioxide and water. (although incomplete burning does cause some by-products like carbon monoxide) Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C8H18) Combustion • Example • • C5H12 + 8 O2 5 CO2 + 6 H2O Write the products and balance the following combustion reaction: • C10H22 + O2 Mixed Practice • 1. 2. 3. 4. 5. State the type, predict the products, and balance the following reactions: BaCl2 + H2SO4 C6H12 + O2 Zn + CuSO4 Cs + Br2 FeCO3 Redox Reactions. Reduction Oxidation GCSE Oxidation: Reduction: •Gain of oxygen •Loss of oxygen •Loss of electrons •Gain of electrons Increase in oxidation number Decrease in oxidation number 4 Experiments: 1. 2. 3. 4. Burning magnesium Copper in silver nitrate solution Chlorine solution and potassium iodide solution Sodium in Water •Word equation •Balanced symbol equation Oxidised – gains oxygen 2Mg(s) + O2(g) 2MgO(s) Must be a redox! Oxidised – loss of e- Mg 2+ Mg Put the e- in. Reduced – gain of e- O +2e- O2- +2e- Cu(s) + 2AgNO3(aq) Cu(NO3 )2(aq) + 2Ag(s) Complete the halfOxidised? equations Reduced? Oxidised – loss of e- Reduced – gain of e- Cu Ag+ +e- Cu2+ +2e- Ag H2(g) + ½ O2(g) H2O(g) Covalent! Need a new definition. No H+ or OH- GCSE Oxidation: Reduction: •Gain of oxygen •Loss of oxygen •Loss of electrons •Gain of electrons Increase in oxidation number Decrease in oxidation number Redox Reactions. Reduction Oxidation Oxidation Numbers The oxidation number of an atom in an element is zero. E.g. Mg in Mg, O in O2. Oxidation Numbers The oxidation numbers of atoms in a compound add up to zero. F -1 O -2 Oxidation state of C in CO2? H +1 ?–4=0 Cl -1 ? = +4 Put the +! Oxidation Numbers The oxidation numbers of atoms in a compound add up to zero. Oxidation state of Mg in MgCl2? +2 F -1 O -2 H +1 Cl -1 Oxidation Numbers The oxidation numbers of atoms in a compound add up to zero. Oxidation state of N in NH3? -3 F -1 O -2 H +1 Cl -1 Oxidation Numbers The oxidation numbers of atoms in an ion add up to the charge on the ion. F -1 O -2 Oxidation state of S in SO42-? H +1 ? – 8 = -2 Cl -1 ? = +6 Oxidation Numbers The oxidation numbers of atoms in an ion add up to the charge on the ion. Oxidation state of S in S2-? -2 F -1 O -2 H +1 Cl -1 Oxidation Numbers The oxidation numbers of atoms in an ion add up to the charge on the ion. Oxidation state of N in NH4+? -3 F -1 O -2 H +1 Cl -1 H2(g) + ½ O2(g) H2O(g) Covalent! Need a new definition. No H+ or OH- GCSE Oxidation: Reduction: •Gain of oxygen •Loss of oxygen •Loss of electrons •Gain of electrons Increase in oxidation number Decrease in oxidation number H2(g) + ½ O2(g) H2O(g) H 0 0 O +1 -2 Covalent! Need a new definition. No H+ or OH- H2(g) + ½ O2(g) H2O(g) H 0 0 +1 -2 O Oxidised? H – increase in oxidation number Reduced? O – decrease in oxidation number