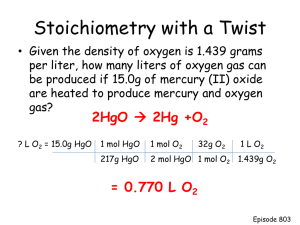35g Br 2 produces 39.5g PBr 3
advertisement

What is the balanced equation for the decomposition of hydrogen peroxide into water and oxygen gas? 2H2O2 2H2O + O2 What does the abbreviation STP stand for? Standard Temperature & Pressure One mole of a compound contains how many particles? 6.02x1023 particles One mole of carbon has a mass of how many grams? 12 grams 6.02x1023 is also known as Avagodro’s Number The sum of the masses of the elements composing a compound is known as its Formula Mass One mole of any gas has what molar volume? 22.4 Liters The mass of each element in a compound divided by the formula mass is the Percent composition What is the percent composition of CO2? %C = 12/44 = 27% %O = 16(2)/44 = 73% Write the balanced formula for the reaction of iron with oxygen gas to form iron (III) oxide. 4Fe + 3O2 2Fe2O3 If 4.3g of iron reacts with oxygen, as in the previous formula, what mass of Fe2O3 would be produced? 6.1 g Fe2O3 4.3g Fe 1mol Fe 56g Fe 2mol Fe2O3 160g Fe2O3 4mol Fe 1mol Fe2O3 How many liters of oxygen are required for the previous reaction? 1.29 L O2 4.3g Fe 1mol Fe 56g Fe 3mol O2 22.4 L O2 4mol Fe 1mol O2 Aluminum reacts with bromine gas to produce aluminum bromide. What is the balanced equation for this reaction? 2Al + 3Br2 2AlBr3 What type of reaction is the one previously written? 2Al + 3Br2 2AlBr3 Synthesis reaction If 15.0g Al reacts with 46.0g Br2, which is the limiting reactant? 46.0g Br2 15.0g Al 46.0g Br2 1mol Al 2mol AlBr3 267g AlBr3 27g Al 2mol Al 1mol AlBr3 1mol Br2 2mol AlBr3 267g AlBr3 160g Br2 3mol Br2 1mol AlBr3 = 148.3g = 51.2g Find the empirical formula for the compound that consists of 20.23% Al and 79.77% Cl. AlCl3 20.23g Al 1mol Al 27g Al 79.77g Cl 1mol Cl 35g Cl = .75 mol Al .75 = 2.28 mol Cl =1 =3 .75 If the molar mass of the compound in the previous question is 264g, what is the molecular formula? Al2Cl6 AlCl3 formula mass = 132 264/132 = 2 For the formula 2P + 3Br2 2PBr3, if 5.0g P and 35g of Br2 react, what is the limiting reactant and how what mass of PBr3 is produced? 35g Br2 produces 39.5g PBr3 5.0g P 35g Br2 1mol P 2mol PBr3 271g PBr3 31g P 2mol P 1mol PBr3 1mol Br2 2mol PBr3 271g PBr3 160g Br2 3mol Br2 1mol PBr3 = 43.7g = 39.5g If the actual yield of PBr3 from the previous problem is 30g, what is the percent yield? 75.9% 30g/39.5g In the writing of an ionic compound, how do we determine the number of each atom in the compound? Criss cross the ionic charges What law are we following when we balance a chemical equation? Law of Conservation of Mass Balance the following equation: Ca(ClO3)2 CaCl2 + O2 Ca(ClO3)2 CaCl2 + 3O2 What is the formula for: iron (III) carbonate? Fe2(CO3)3 Write the balanced equation for the following word equation: Aluminum chloride + Calcium Aluminum + Calcium chloride 2AlCl3 + 3Ca 2Al + 3CaCl2