Equation Match Up Activity
advertisement

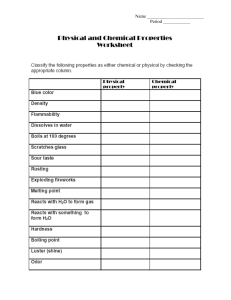
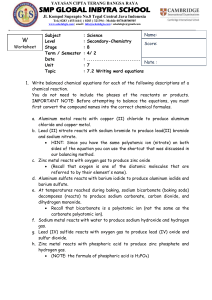
Unit 6: Equation Match Up Name(s): _____________________________________ Date: _____________________________________ Block: _____________________________________ Outcomes and Expectations Determine the formula of compounds based on their names Determine the skeleton chemical equation based on the names of the compounds involved Instructions 1. 2. 3. 4. 5. 6. Pick the written out version of a chemical reaction from a bag. Determine the chemical formulas for each substance in the written form of the reaction. Using the formulas and the symbols in a chemical equation construct the chemical equation. Use the pieces “reactants” and “products” to label each part of the equation. Have your teacher double check your equations. Write the equation for the corresponding reactions in the spaces below. 1. Aluminum metal reacts with Chlorine Gas and forms Aluminum Chloride. 2. Sodium Carbonate reacts with Magnesium Phosphate to create Magnesium Carbonate and Sodium Phosphate. 3. Chlorine gas reacts with Sodium Bromide and creates Sodium Chloride and Bromine gas. 4. Iron metal reacts with Copper (II) Sulfate to create Iron (II) Sulfate and Copper metal. 5. Hydrogen Peroxide decomposes to Water and Oxygen gas. 6. Methane is combusted in the presence of Oxygen Gas and creates Water vapor and Carbon Dioxide. 7. Silver Nitrate reacts with Hydrochloric Acid to form Nitric Acid and Silver Chloride. 8. Copper metal and Silver Nitrate react to form Silver metal and Copper (I) Nitrate. 9. Calcium reacts with Sulfur gas to form Calcium Sulfide. 10. Bromine Gas reacts with Potassium Iodide to form Iodine Gas and Potassium Bromide. 11. Sucrose reacts with Oxygen gas and creates Water and Carbon Dioxide. 12. Nitric Acid decomposes to form Nitrogen Dioxide, Water, and Oxygen gas.