Solubility Rules & Reference Tables
advertisement

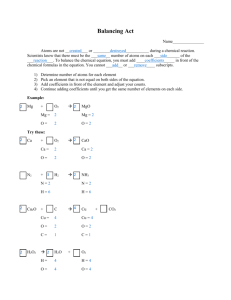
Balancing Equations Chemical Reactions Chemical rxns occur when bonds (between electrons of atoms) are formed or broken Chemical rxns involve: changes making new materials with new properties energy in the chemical composition of matter changes: Bond breaking absorbs Energy (endothermic) Bond making releases Energy (exothermic) Note: The overall change in energy for certain rxns can be found on Ref. Table I Chemical Equations Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al (s) + 3 O2 (g) → 2 Al2O3 (s) The numbers in the front are called stoichiometric coefficients Symbols Used in Equations Solid (s), Liquid (l), Gas (g) Aqueous solution (aq) (dissolved in water) Catalyst H2SO4 Escaping gas () Change ( or Pt of heat energy or + 3kJ or – 3kJ) *there is no subtraction…a negative sign means released/exothermic Express a chemical equation as follows: Reactants Products The arrow is equivalent to an “=“ math. When describing the equation use the word “yields” or “produces” instead of equals Ex: C + O2 CO2 This reads “carbon plus oxygen react to yield carbon dioxide” Because of the principle of the conservation of matter an equation must be balanced. matter can’t be created or destroyed Must have the same number of each type of atom on both sides. Must have same # grams of matter on both sides!! Lavoisier, 1788 Coefficients not Subscripts When balancing add coefficients in front of compounds to balance the atoms in the reaction DO NOT change the subscripts of the formulas. Changing subscripts changes the compound. Subscripts: tell you how many atoms of a particular element are in a compound. Coefficient: tells you about the quantity, or number, of molecules of the compound. Balancing a chemical equation: DO NOT CHANGE THE FORMULAS! Find number of atoms for an element on left side. Compare those against number of the atoms of the same element on right side. Place coefficients in front of formulas so that left side has the same number of atoms as the right side for EACH element Check your answer to see if: The numbers of atoms on both sides of the equation balanced. The coefficients are in the lowest possible whole number ratios. Helpful hints for balancing equations: Take one element at a time, working left to right. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, double all the coefficients and try again. Useful in combustion reactions! (Shortcut) Polyatomic ions that appear on both sides of the equation should be balanced as independent units Just circle them and think of them as one thing Balancing Equations ___ H2(g) + ___ O2(g) → ___ H2O(l) What happened to the other Oxygen atom????? This equation is not balanced! Balance Equation Na + Cl2 Na = 1 Cl = 2 NaCl Na = 1 Cl = 1 The number of sodium atoms balance but the chlorine does not. Use coefficients in order to balance this equation. Inserting Coefficients Na + Cl2 2 NaCl Na = 1 Cl = 2 Na = 2 Cl = 2 Now chlorine balances but the sodium does not! So we go back and balance the sodium. Finally balanced! 2Na + Cl2 2 NaCl Na = 2 Cl = 2 Na = 2 Cl = 2 Since the number of each element on the reactant side and the product side of the equation are equal, the equation is balanced. Balance Practice (Page 2 in Chemical Equations Packet) ___Al2O3 + ___H2 → ___H2O + ___Al ___MgI2 + ___K → ___KI + ___Mg ___AlF3 → ___Al + ___F2 Balance Practice (Page 2 in Chemical Equations Packet) ___Na2CO3 + ___Mg → ___MgCO3 + ___Na ___ZnO → ___Zn + ___O2 ___Fe + ___O2 → ___Fe2O3 Balancing Practice For more help go to: http://richardbowles.tripod.com/chemistry/ balance.htm#part0 For some fun balancing equations go to: http://www.mpcfaculty.net/mark_bishop/b alancing_equations_tutorial.htm