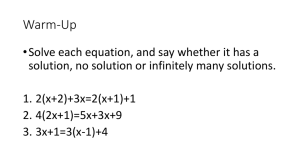Agenda: 11/19 * Types of Chemical Reactions
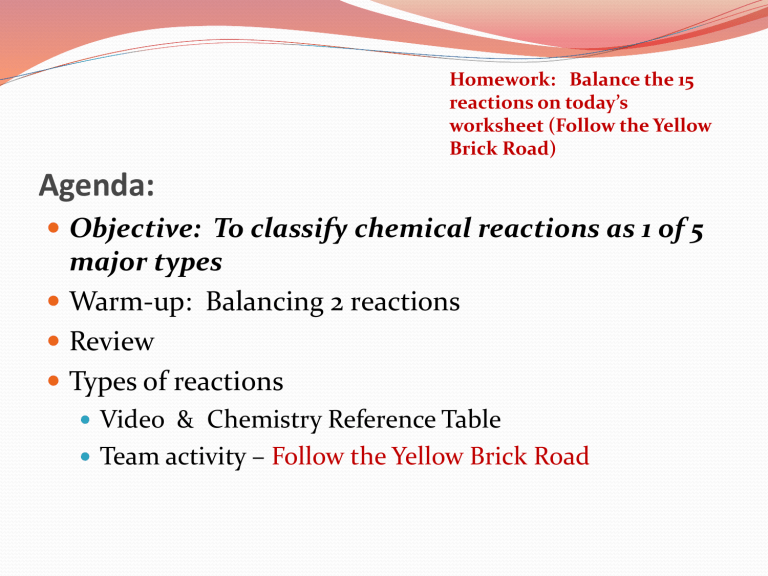
Homework: Balance the 15 reactions on today’s worksheet (Follow the Yellow
Brick Road)
Agenda:
Objective: To classify chemical reactions as 1 of 5 major types
Warm-up: Balancing 2 reactions
Review
Types of reactions
Video & Chemistry Reference Table
Team activity – Follow the Yellow Brick Road
Overall Objective: To predict the products in a chemical reaction
What is a chemical reaction?
How are the chemical reactions represented?
What are the amounts needed in a chemical reaction?
What is usually produced in a chemical reaction?
Types of reactions (typical products )
Overall Objective: To predict the products in a chemical reaction
What is a chemical reaction?
Rearrangements of atoms to form different substances
Involves breaking and forming of chemical bonds
How are the chemical reactions represented?
Chemical equations; symbols
What are the amounts needed in a chemical reaction?
Balancing equations
What is commonly produced in a chemical reaction?
Types of reactions (typical products )
Warm-up: Word Equations for Chemical Reactions
#12
Magnesium + nitric acid hydrogen magnesium nitrate +
#16
Sodium hydroxide + hydrochloric acid sodium chloride + water
FIVE BASIC WAYS TO CLASSIFY CHEMICAL
REACTIONS:
1.
2.
3.
4.
5.
Synthesis
Decomposition
Single-Replacement
Double-Replacement
Combustion
Classifying reactions – 5 types
1.
2.
Work as a Team
Resources: video clip; reference tables; worksheet
3.
1.
2.
What distinguishes each type of reaction?
Differences for each type (reference tables)
Write a short explanation
4.
1.
Classify 15 reactions
3 per type
5. Show your team’s work on large white boards
10 Youtube videos on chemical reactions
5 Types of Chemical reactions
http://www.youtube.com/watch?v=i-
HHvx1VC_8&list=PL9B92AB1CD71DA186
Follow the Yellow Brick Road
Group 1
___ ___ ____
Explanation:
Group 2
___ ___ ___
Explanation:
Group 3
____ ____ ____
Explanation:
Reaction type: Reaction type: Reaction type:
Group 4
____ ____ ____
Explanation:
Group 5
____ ____ ____
Explanation:
Reaction type Reaction type