10.3 – phase change lab KEY
advertisement

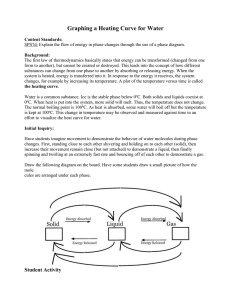
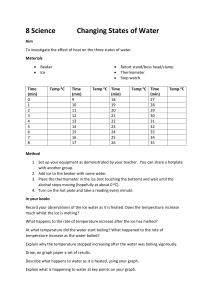
Solid Liquid Gas Can’t change shape or volume Can change shape not volume Can change shape and volume freezing -liquid to solid melting -solid to liquid condensation -gas to liquid vaporization/boiling sublimation -liquid to gas -solid to gas Temperature measures the Kinetic Energy (speed) of the particles in a substance. As you add heat to a substance the particles will move faster. o Water boils at 100 C and ice water exists at o 0 C. Heating Curve of Water o Temperature ( C) --> 1 square = 5 C Hold paper sideways, holes on top Time (s) --> 1 square = 30 seconds O Heating Curve of Water 120 100 80 60 Water Temp (degrees Celcius) 40 20 0 0:00 -20 1:12 2:24 3:36 4:48 6:00 7:12 8:24 9:36 10:48 12:00 Sources of error could be calibration of thermometer, purity of the water, the placement of the thermometer, how you stirred,. Water can boil at temps greater than O 100 C if there are impurities in the water. Another possibility is a miscalibrated thermometer (When ice melts or water boils) The temperature of a substance going through a phase change will remain constant. (When there was only water!) The temperature increased when there was only one phase present in the beaker. While the ice is melting, the energy is weakening the intermolecular forces causing a phase change. When only water is in the beaker, the energy speeds up the water molecules, which increases temperature. When the water is boiling the energy is weakening the intermolecular forces causing a phase change.