Carbonate Sedimentary Rocks: Classification & Formation
advertisement
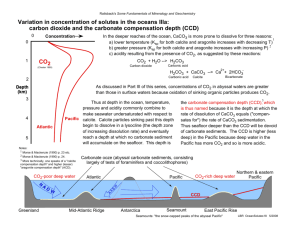
Chapter 6: Carbonate Sedimentary Rocks There are two main categories of carbonate rocks: • Calcite (CaCO3) • Dolomite (CaMg(CO3)2) Both Calcite and Dolomite will contain varying amounts of Calcium and Magnesium. Other elements make up only trace amounts. Calcite Dolomite Calcite Group Iceland Spar CaCO3 Rhodochrosite MnCO3 Magnesite MgCO3 Siderite FeCO3 Smithsonite ZnCO3 Dolomite Group Dolomite CaMg(CO3)2 Ankerite Ca(Mg,Fe,Mn)(CO3)2 Aragonite Group Aragonite CaCO3 Strontianite SrCO3 Cerussite PbCO3 Witherite BaCO3 Limestone (composed of primarily CaCO3) textures Carbonate Grains • Carbonate clasts (extraclasts & intraclasts--Lithoclasts) •Extraclast: derived from older limestone located outside the depositional environment. •Intraclast: derived from seafloor, adjacent tidal flats or a carbonate beach •Lithclast: a nonspecific term used when the distinction between extra & intraclast cannot be made. • Skeletal particles • Ooids • Peloids • Aggregate Grains Matrix cements: either sparry calcite or micrite A: Rounded clasts cemented by sparry calcite. B: Angular clasts in micrite. C: Fossiliferous limestone with sparry cement. D: Normal ooids cemented with sparry. E: Radial ooids cemented with sparry & micrite. F: Pellets cemented with sparry. Ooid Aggregate grain (Grapestone) Microcrystalline calcite (Micrite) versus Sparry calcite Classification of Carbonate Rocks Limestone classification based on textures Origin of Carbonate Rocks Limestone: CO2 + H2O ↔ H2CO3 (carbonic acid) H2CO3 ↔ H+ + HCO3- (bicarbonate ion) HCO3- ↔ H+ + CO32- (carbonate ion) __________ H2O + CO2 + CaCO3 ↔ Ca2+ + 2HCO3(where the CaCO3 can be either Calcite or Aragonite) Principle factors that affect inorganic precipitation of CaCO3 in water (Table 6.4 pg. 175) Water condition Direction of change Directed effect Effect on CaCO3 solubility Kind of CaCO3 precipitated Temperature Increase Loss of CO2, increase in pH More likely to precipitate Micrite or ooids Pressure Decrease Loss of CO2, increase in pH More likely to precipitate Micrite or ooids Salinity Decrease Decrease in More likely to activity of precipitate “foreign cations” Micrite or ooids Organic activity and CaCO3 precipitation •Extraction of CaCO3 from water Growth of shells and tests •Photosynthesis Removes CO2 from water, thereby increasing pH. •Decay of soft tissue Increases pH of water •Feeding, sediment ingestion Reshapes sediment •Bacterial activity Promotes CaCO3 precipitation Era Period Dominate Carbonate Mineral Ceno-zoic Neogene-Quaternary A + HMC (Aragonite Sea) Paleogene Mesozoic Cretaceous Low-magnesian Calcite (LMC) (Calcite Sea) Jurassic Triassic Permian Pennsylvanian Aragonite (A) + Highmagnesian Calcite (HMC) (Aragonite Sea) Paleozoic Mississippian Devonian Silurian Ordovician Cambrian Low-magnesian Calcite (LMC) (Calcite Sea) Calcite versus Aragonite Dolomite Classification and Variation Dolomite formation: The Dolomite problem…. Scientists have not yet been successful in the laboratory in precipitation perfectly ordered Dolomite (50% Calcium and 50% Magnesium) at the normal temperatures and pressures of the Earth’s surface. Ca2+(aq) + Mg2+(aq) + 2CO32-(aq) = CaMg(CO3)2(solid) 2CaCO3(solid) + Mg2+(aq) = CaMg(CO3)2(solid) + Ca2+(aq) Sabkha Environment Carbonate Diagenesis Carbonate Diagenesis continued… Stylolites: a pressure-solution feature common in carbonate rocks. These features are often associated with clay minerals and other fine-size noncarbonate minerals that accumulate as carbonate minerals dissolve.