Natural History of Compensated Cirrhosis in Patients with
advertisement

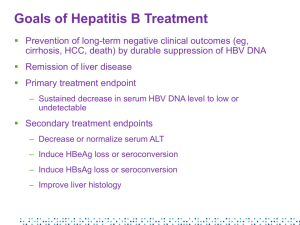
Introduction and Program Overview Eugene R. Schiff, MD, MACP, FRCP, MACG, AGAF Program Chair Leonard Miller Professor of Medicine University of Miami School of Medicine Miami, Florida Program Overview • Chronic hepatitis B (HBV) infection is significantly underdiagnosed and under-treated in the US • Much new data has emerged, increasing our knowledge of the natural history of this disease and its treatment • Effective new anti-HBV agents and novel treatment approaches for long-term management are now in use • Interactive case presentations will help us review the latest developments in the understanding and treatment of the disease Educational Objectives Upon completion of this activity, participants should be able to: • DESCRIBE the epidemiology and natural history of hepatitis B virus (HBV) infection • IMPLEMENT an activity of screening, vaccination, and diagnosis of HBV within their clinical practices • EVALUATE the risks and benefits of available agents for treating chronic HBV infection • EVALUATE current data on the potential use of combination therapy for patients with chronic HBV infection Agenda • The Hepatitis B Virus: A Silent Killer • Whom to Treat/When to Treat • Treatment Options for Chronic HBV Infection • Combination Therapy: Controversies and Uncertainties Program Faculty Program Chair Eugene R. Schiff, MD, MACP, FRCP, MACG, AGAF Leonard Miller Professor of Medicine Director, Schiff Liver Institute Director, Center for Liver Diseases Division of Hepatology University of Miami School of Medicine Miami, FL Norah A. Terrault, MD Associate Professor of Medicine Director, Viral Hepatitis Research in Liver Transplantation Dept of Medicine, Division of Gastroenterology University of California, San Francisco San Francisco, CA Marion Peters, MD, FRACP Professor of Medicine Chief of Hepatology Research University of California, San Francisco San Francisco, CA Tram T. Tran, MD Assistant Professor of Medicine Geffen UCLA School of Medicine Division of Gastroenterology Medical Director of Liver Transplant Comprehensive Transplant Center Cedars Sinai Medical Center Los Angeles, CA Mark Sulkowski, MD Associate Professor of Medicine Medical Director, Viral Hepatitis Center Johns Hopkins University School of Medicine Baltimore, MD Audience Participation • Audience Response System Used to pose a series of questions during the meeting At slide prompts, key in your answers on the keypads Please return your keypad at the end of the program • Questions? Question cards • Please jot down your questions, and staff will pick them up during the course of the meeting Microphones Accreditation Statement • This activity has been planned and implemented through the joint sponsorship of the University of Kentucky College of Medicine and HealthmattersCME • The University of Kentucky College of Medicine designates this educational activity for a maximum of 2.0 AMA PRA Category 1 Credits™ How to Obtain CME Credit • Complete this activity in its entirety • After the activity, go to www.cecentral.com/getcredit • Enter activity code MLN09103 • Log in or register for a free account • Complete activity evaluation and get credit • A printable certificate will be issued Disclosure Statements Program faculty have disclosed their relevant financial relationships with commercial interests that produce health care goods and/or services consumed by, or used on patients. Written disclosures can be found within your folder. The Hepatitis B Virus: A Silent Killer Tram T. Tran, MD Assistant Professor of Medicine Geffen School of Medicine at UCLA Medical Director of Liver Transplant Comprehensive Transplant Center Cedars-Sinai Medical Center Los Angeles, California Case Presentation • A 44-year-old Russian man who immigrated to the United States in 1989 is seeing you for abnormal transaminase levels. - ROS: occasional flares of diarrhea and abdominal pain, currently asymptomatic - PMH: Crohn’s disease; previously vaccinated for hepatitis B virus - Social: 1-2 drinks per week, works construction - Family Hx: Mother d. cirrhosis; was “drinker” - Meds: 5-aminosalicylic acid (5-ASA) - PE: normal Let’s Vote! Audience Response Question New CDC 2008 Guidelines recommend HBV screening in immigrants from endemic areas with hepatitis B prevalence of: 14% A. > 25% 26% B. >10% 24% C. >8% 36% D. >2% Hepatitis B: Region/Country Region Countries Africa All countries Asia All countries Australia & South Pacific All countries except Australia and New Zealand Middle East All countries except Cyprus and Israel Eastern Europe All countries except Hungary Western Europe Malta, Spain, and indigenous populations of Greenland North America Alaska and indigenous populations of northern Canada Mexico and Central America Guatemala and Honduras South America Ecuador, Guyana, Suriname, Venezuela, Amazonian areas Caribbean Antigua, Dominica, Granada, Haiti, Jamaica, St Kitts and Nevis, St Lucia, Turks and Caicos CDC.MMWR 2008 Incidence* of Acute Hepatitis B, by Age Group, Sex, and Year – United States, 1990-2002 20 Males aged 0-19 yr Males aged 20-39 yr Males aged ≥40 yr Females aged 0-19 yr Females aged 20-39 yr Females aged ≥40 yr Total Incidence 16 12 8 4 0 1990 1992 1994 1996 Year *Per 100,000 population. 1998 2000 2002 Hepatitis B: Disease Progression Liver Cancer (HCC) 5%-10% 2%-6% Acute Infection Chronic Infection 90% in perinatal 30%-90% in children <5 years old 5% in healthy adults Higher in HIV, immunosuppressed Cirrhosis Liver Transplantation Death 10%-30% Liver Failure (Decompensation) 23% within 5 years Chronic HBV is the 6th leading cause of liver transplantation in the US Torresi J et al. Gastroenterology. 2000;118(2 Suppl 1):S83-S103. Fattovich G et al. Hepatology. 1995;21(1):77-82. Moyer LA et al. Am J Prev Med. 1994;10(Suppl):45-55. Perrillo RP et al. Hepatology. 2001;32(2):424-432. Asian-American Age-Adjusted Liver Cancer Rates (California, 2000-2002) Incidence Male Female 54.3 Rate (per 100,000) Rate (per 100,000) Male Female Mortality 33.7 23.3 16.8 15.8 15.9 9.3 8.1 7.6 5.4 35.5 26.6 19.9 12.0 Filipino Vietnamese Korean Japanese 11.5 8.3 7.8 7.8 6.8 4.2 2.5 Chinese 10.4 White Chinese Filipino 2.7 Vietnamese Korean Approximately 3.7 million Asians in California. Cancer data from California Cancer Registry. McCracken M et al. CA Cancer J Clin. 2007;57:190-205. 6.0 Japanese White Case Presentation: Laboratory Findings • • • • • • • • CBC: WBC 5.5, Hgb 12.5, Plt 288 AST 39 IU/L, ALT 35 IU/L Bilirubin 1.0 mg/dL, INR 1.1, albumin 3.7 g/dL HBsAg: positive HBeAg: negative Anti-HBe: positive HBV DNA 1800 IU/mL HCV, HIV, HDV negative Let’s Vote! Audience Response Question This patient is most likely in which stage of CHB infection? 18% 8% 38% 21% 15% A. B. C. D. E. Immune tolerant Immunoactive/immune clearance Inactive carrier HBeAg CHB Can’t tell Phases of HBV Infection Yim JY, Lok AS-F. Hepatology. 2006;43:S173-S181. Case Presentation (cont’d) • Serial follow-up of his liver tests reveals - ALT fluctuation 30105 - HBV DNA 1800 IU/mL 7600 IU/mL ALT and Histology • 192 patients (Boston) • HBV DNA > 10,000 copies/mL • Liver biopsy data • Stratified by ALT - Persistently normal (< 40 IU/L), n=59 - 1-1.5 x ULN, n=26 - >1.5 x ULN, n=107 Lai M et al. J Hepatol. 2007;47(6):760-767. Grade of Inflammation by ALT Group 78% 70% Percentage Grade 0 54% 60% Grade 1 50% 40% 34% Grade 2 Grade 3 30% 20% 10% 0% PNALT ALT 1-1.5 ULN PNALT, persistently normal ALT. Lai M et al. J Hepatol. 2007;47(6):760-767. ALT >1.5 ULN Stage of Fibrosis by ALT Group 62% 18% 34% Case Presentation (cont’d) • Liver biopsy is performed: - Grade 2-3 inflammation - Stage 2 fibrosis HBeAg Seroconversion • 298 patients with documented HBeAg seroconversion - 116 treatment induced, 182 spontaneous • Reactivation in 71 patients (39%) - Older age, male gender, and higher ALT at seroconversion were risks for reactivation (all P <.006) - No difference between interferon, adefovir, lamivudine treatment • Treatment-induced seroconversion less durable than spontaneous - Remission of ALT shorter (14 vs 22 months, P=.037) - More likely to have HBeAg reactivation at 48 months (38% vs 25%, P=.048) Lim G et al. 58th AASLD; 2007; Boston. Poster 937. HBeAg Seroconversion to Anti-HBe • Development of cirrhosis complications and HCC - 3233 Chinese patients - Mean follow-up 46.9 months Median age (yr) Percentage anti-HBe 35 - All complications 57.2 73.5 Ascites 57.7 68.8 Spontaneous bacterial peritonitis 60.0 76.7 Varices 54.3 76.3 Encephalopathy 58.5 65.0 Hepatocellular carcinoma 59.0 81.1 HBeAg seroconversion Yuen MF et al. Gut 2005;54(11):1610-1614. Incidence of Cirrhosis: HBeAg Status Taiwan and Korea Europe 50 50 HBeAg negative HBeAg positive 40 30 Percent Percent 40 20 30 20 10 10 0 0 0 1 2 3 HBeAg negative HBeAg positive 4 Years Fattovich G et al. J Hepatol. 2008;48(2):335-352. 5 0 1 2 Years 3 4 5 Cumulative Incidence of Hepatocellular Carcinoma (HCC) Taiwan, China, Singapore, Korea and Japan Europe and USA 20 20 Cirrhosis Chronic hepatitis Inactive carrier 15 10 Percent Percent 15 5 10 5 1 1 0 0 0 1 2 3 Cirrhosis Chronic hepatitis Inactive carrier 4 Years Fattovich G et al. J Hepatol. 2008;48(2):335-352. 5 0 1 2 Years 3 4 5 AASLD Guidelines: Periodic Screening for HCC • At-risk hepatitis B carriers - Asian males >40 years of age Asian females >50 years of age All cirrhotic hepatitis B carriers Family history of hepatocellular carcinoma Africans >20 years of age Those with high HBV DNA levels and those with ongoing hepatic inflammatory activity remain at risk for hepatocellular carcinoma • Liver ultrasound every 6 to 12 months Bruix J et al. Hepatology. 2007;42:1208-1236. Case Presentation (cont’d) • Patient’s Crohn’s disease flares; consideration is made for steroids and possibly anti-tumor necrosis factor (TNF) therapy Let’s Vote! Audience Response Question Is it necessary to screen patients for HBV before anti-TNF therapy? 68% 28% 3% 2% A. Yes, screen all patients B. Yes, but only those with risk factors for HBV C. No, just monitor D. No, never Screening: New CDC Guidelines • CDC Guidelines 2006 - Persons born endemic areas >8% prevalence - Pregnant women, infants - Sexual, household contacts of HBV+ - HIV - Needlestick/assault - Hemodialysis patients - Blood donors • CDC Guidelines 2008 - Persons born endemic areas >2% prevalence - US-born children of immigrants from highrisk areas - Injection drug users - MSM - Immunosuppressive Rx • GI, rheumatologic, oncologic, tx - ALT/AST elevation Centers for Disease Control; MMWR Sept 19 2008 Case Presentation (cont’d) • Patient started on antiviral therapy prior to antiTNF treatment • HBV DNA becomes undetectable • ALT remains persistently normal Summary • HBV burden is significant, some groups disproportionately affected • New CDC guidelines have broadened screening recommendations • Disease progression may be independent of biochemical and serological markers Whom to Treat When to Treat Norah Terrault, MD, MPH Associate Professor of Medicine Director of Viral Hepatitis Research in Liver Transplantation University of California, San Francisco San Francisco, California Goals of Treatment Improved histology Anti-HBe+ Anti-HBs+ Improved survival Loss of HBsAg Loss of HBeAg Loss of HBV DNA HBV is controlled not eradicated TIME Decision to Treat: Balancing Benefits and Risks Risk of Liver Complications Costs Side Effects Drug Resistance Factors Associated With Disease Progression in Patients With CHB Host Factors Virus Factors • >40 years of age • High serum HBV DNA concentrations • Male • Immune status • Prolonged time to HBeAg seroconversion • Development of HBeAg(-) chronic hepatitis • Core promoter HBV variant • Genotype C Yim HJ, Lok ASF. Hepatology. 2006;43:S173-S181. Environmental Factors • Concurrent infection (HCV, HDV, HIV) • Alcohol consumption • Diabetes mellitus • Obesity Persistent Elevated HBV DNA and Cumulative Incidence of HCC P < .001 Adjusted HR for HCC (95% CI) 12 67% ≥40 yrs and 62% male 10.1 (6.3-16.2) 10 P < .001 7.3 8 (3.5-15.3) P < .001 6 3.8 (1.7-8.4) 4 P = NS 2 0 1 26/2034 < 104 HBV DNA : Baseline Follow-up (copies/mL) Not Tested Chen CJ et al. JAMA. 2006;295(1):65-73. 8/146 ≥105 < 104 10/120 ≥105 → 104 - < 105 55/537 ≥105 ≥105 Case Presentation • A 37-year-old Asian woman is referred for HBsAgpositive status. Discovered when mother was diagnosed with HCC at age 65 • Asymptomatic, recently married, husband vaccinated, no children • No medications other than oral contraceptives • Initial lab results: - HBeAg+, HBV DNA 2.5 million IU/mL - ALT 30 IU/L, AST 27 IU/L, total bilirubin 0.6 mg/dL, albumin 4.0, g/dL, INR 1.0, platelets 300K Case Presentation (cont’d) • Ultrasound findings: - Normal-appearing liver with normal echotexture, no splenomegaly or collaterals • Repeat labs: - 3 months: ALT 35 IU/L - 6 months: ALT 31 IU/L, HBV DNA 1.3 million IU/mL Let’s Vote! Audience Response Question What do you recommend at this point? A. Continue monitoring ALT every 3-6 months and treat if ALT increases to ≥2 X ULN 4% B. Obtain HBV genotype and treat if genotype A 25% C. D. Start treatment, regardless of HBV genotype Obtain liver biopsy and treat if significant inflammation or fibrosis 25% 45% Recommendations: Whom to Treat AASLD Guidelines 2007 • Treatment indicated for ‘active’ disease: - ALT ≥2 ULN - HBV DNA ≥20,000 IU/mL Or if - ALT 1-2 ULN and ≥age 40, consider biopsy and treat if significant fibrosis or necroinflammation is present Recommendations: Whom to Treat: NIH 2008 HBV Consensus Statement Treatment Indicated • Fulminant and decompensated disease • Cirrhosis with elevated HBV DNA • Chemotherapy or other IMS therapy • (Liver transplantation) Treatment May be Indicated • Immune active phase • Reactivation phase Treatment Not Indicated • Immune tolerant • Immune inactive • Latent HBV NIH Consensus Development Conference: Management of Hepatitis B. Draft Statement. October 22, 2008 5:50 PM; http://consensus.nih.gov/2008/hepB Recommendations: Whom to Treat AASLD Guidelines 2007 • HBV DNA ≥20,000 IU/mL • ALT ≥2 ULN • If ALT 1-2 ULN and ≥age 40, consider biopsy and treat if significant fibrosis or necroinflammation EASL Guidelines 2009 • HBV DNA ≥ 2,000 IU/mL • ALT ≥ ULN “Gray Areas” • ALT cutoff - New “normal” ULN for ALT - Is ≥2 ULN appropriate? • Biopsy criteria - Significant? ≥G2 or G3, ≥F2? • HBV viral load - Differs for HBeAg negative vs positive CHB? Patients With CHB With Significant Liver Disease (ALT<2 ULN and HBV DNA >105 copies/mL) ALT<2 ULN With Laboratory Cutoff ALT<2 ULN With Revised ALT ULNs* n=451 n=141 HBeAg(+) CHB Necroinflammation ≥7 Fibrosis ≥4 65% 13% 68% 11% HBeAg(-) CHB Necroinflammation ≥7 Fibrosis ≥4 71% 8% 75% 17% * <30 IU/L males, <19 IU/L females Terrault NA et al. Digestive Disease Week; 2007; Washington, DC. Chronic HBV Infection and Normal ALT: Summary of Recent Literature If focus on fibrosis: • Range 8% to 47% of patients have stage 2 fibrosis or greater • Normal ALT levels often on follow-up • Factors associated with higher fibrosis - Age >35 yr - Male gender - Level of ALT Yang LM et al. Chinese J Dig Dis. 2002;3:150-153. Tsang PSY et al. Clin Gastroenterol Hepatol. 2008;6:569-574. Kumar J et al. Gastroenterology. 2008;134:1376-1384. Wang C et al. Hepatology. 2005;42:573A. Lai M et al. Hepatology. 2005;42:720A. Terrault NA et al. Gastroenterology. 2007;132:A72. [#94] Let’s Vote! Audience Response Question Based upon the AASLD Guidelines, which of the following HBeAg-positive profiles warrants treatment? A. ALT 45, HBV DNA 50,000 IU/mL, no biopsy available B. ALT 45, HBV DNA 500 million IU/mL, biopsy 14% shows F0, G1-2 disease C. ALT 18, HBV DNA 22 million IU/mL, no 3% biopsy D. ALT 45, HBV DNA 57,000 IU/mL, biopsy 71% shows F2, G2-3 disease 12% HBeAg-Negative HBV Disease: Diagnostic Dilemmas Anti-HBe positive HBV DNA ALT Histology Active HBeAgNegative Disease Inactive Chronic HBV >20,000 IU/mL HBV DNA <2000 IU or negative Elevated Normal Significant inflammation and fibrosis Inactive hepatitis with variable fibrosis Fluctuating Course of HBeAgnegative Chronic Hepatitis B 400 With flares 300 73 pts (44.5%) and normalization 200 Asymptomatic flare-up: 90% of cases 100 0 A L T 400 Without flares 300 59 pts (36.0%) 200 100 Flare-up yearly frequency: once 57.1% twice 20% < once 22.8% 0 400 With flares and without normalization 300 200 32 pts (19.5%) 100 0 0 12 months 24 Brunetto MR et al, J Hepatol 2002 Biochemical patterns in 164 untreated patients after 23 months (range 12-36) monthly monitoring Let’s Vote! Audience Response Question Based upon the AASLD Guidelines, which of the following HBeAg-negative profiles warrants treatment now? 3% 41% 21% 35% A. B. C. D. ALT 60, HBV DNA 500 IU/mL, no biopsy available ALT 40, HBV DNA 8000 IU/mL, biopsy shows G2, F2, no steatosis ALT 40, HBV DNA 200,000 IU/mL, biopsy shows G0-1, F0-1 fibrosis, G2 steatosis ALT 20, HBV DNA 100 IU/ml, biopsy shows F4 (cirrhosis) CHB Treatment Algorithm for Cirrhotic Patients Treatment Criteria Recommended Action If HBV DNA ≥2000 IU/mL, any ALT OR If HBV DNA <2000 IU/mL, elevated ALT Treat If HBV DNA<2000 IU/mL and normal ALT Observe Keeffe EB et al. Clin Gastroenterol Hepatol. 2006;4(8):936-962. Lok AS, McMahon BJ. Hepatology. 2007;45(2):507-539. Considerations in Applying Treatment Guidelines • HBV viral load - HBeAg-negative: if 2000-20,000 IU/mL, may benefit from additional testing to determine disease severity • ALT cutoff - Use “normal” ULN for ALT - Lack of ALT elevation does not exclude significant disease, though advance fibrosis infrequent • Cirrhosis - Levels of HBV DNA differ, any ALT Case Presentation (cont’d) • You perform a liver biopsy, which shows grade 2 necroinflammation and stage 2 fibrosis • Additional laboratory testing: - HBV genotype B • Patient informs you that she and her husband would like to start a family within the year Let’s Vote! Audience Response Question What do you recommend? 16% A. 20% B. 28% C. 37% D. Defer treatment until after delivery of infant Deferral of pregnancy to undergo treatment with peg-IFN for 24 weeks Proceed with pregnancy but add lamivudine in last trimester for prevention of perinatal transmission Start treatment now with tenofovir HBV Treatment and Pregnancy • If can defer, this is often best strategy • If treating in pregnancy: - Lamivudine is treatment of choice, if limited duration Pregnancy category C drug with long safety record in HIV+ women - Tenofovir and telbivudine Pregnancy category B drugs Tenofovir has accumulating safety record in HIV+ women - Risk-benefit needs to be individualized - Antiviral therapy in last trimester may reduce perinatal transmission if mother has high HBV DNA (>107-8 IU/mL) van Zonneveld et al. J Viral Hepat. 2003(4);10:294-297. Hepatitis B Treatment: Summary • Chronic HBV is dynamic disease - Regular monitoring needed to determine timing of treatment and other interventions • Primary determinants of treatment initiation are - HBV DNA level ≥20,000 IU/mL - ALT level ≥2 ULN - ± Histological severity of disease • Assessment of histology most helpful in borderline ALT and HBV DNA cases • Cirrhotics: lower thresholds to treat Treatment Options for Chronic Hepatitis B Infection Mark Sulkowski, MD Associate Professor of Medicine Divisions of Infectious Diseases and Gastroenterology/Hepatology Johns Hopkins University Baltimore, Maryland Case Presentation • A 58-year-old man from Malaysia is referred for evaluation – No comorbid conditions – He reports that his mother died of liver cancer – He was initially diagnosed ~ 1999 and treated for about 3 months with “a pill” • Evaluation – – – – – AFP = 9 ng/mL HBeAg + ALT = 64 (< 40 U/L) HBV DNA = 28.8 million IU/mL Liver CT scan = “normal” Let’s Vote! Audience Response Question Which of the following evaluations may be helpful for guiding HBV treatment decisions? 6% 7% 24% 6% 57% A. B. C. D. E. HBV genotype Resistance testing Liver biopsy ALT level All of the above Case Presentation (cont’d) • Liver biopsy was performed – Chronic portal inflammation with mild focal lobular hepatitis – Portal and septate fibrosis with ill-defined focal parenchymal nodularity • HBV genotype C • PCR amplification and DNA sequencing reveal polymorphism at position 204 (M → V) Goal of Anti-HBV Therapy • Improve QOL and survival by preventing progression to cirrhosis, end-stage liver disease, hepatocellular carcinoma and death • Mechanisms to achieve this goal – Immune control of HBV replication: seroconversion – Antiviral control of HBV replication: long term suppression • Eradication is not possible, due to cccDNA EASL Clinical Practice Guidelines. J Hepatol. 50(2009),doi:10.1016/j.jhep.2008.10.001. Recommended First-Line HBV Treatment: Peg-IFN, ETV, TDF Approved for HBV • Interferon alfa • • - Interferon alfa-2b - Peginterferon alfa-2a Nucleoside analogues - Lamivudine* - Telbivudine - Entecavir Nucleotide analogues - Adefovir - Tenofovir DF* Unlabeled • Emtricitabine* • Combination therapy - PegIFN + nucleos(t)ide analogue - Nucleoside + nucleotide analogue* *Approved by the FDA for treatment of HIV infection First-line agents in guidelines: Keeffe EB, et al. Clin Gastroenterol Hepatol. 2008 Aug 23. [Epub ahead of print]; EASL Clinical Practice Guidelines. J Hepatol. 50(2009),doi:10.1016/j.jhep.2008.10.001. Let’s Vote! Audience Response Question A 55-year-old man - Genotype C HBV DNA = 28.8 million IU/mL ALT = 54 Histologic evidence of cirrhosis Which of the following characteristics is/are associated with a favorable response to interferon? 3% 23% 53% 15% 6% A. B. C. D. E. Male sex Genotype C Elevated ALT High HBV DNA level Cirrhosis Peg-IFN for Chronic Hepatitis B Peg-IFN • Finite duration • No resistance • Higher rates of seroconversion • Poor tolerance • SQ injection • High ALT activity1,2,3 • Low baseline serum HBV DNA concentration4 • Genotype A or B5,6 • Absence of comorbidities • No cirrhosis • No decompensated liver disease 1. Piratvisuth T, et al. Hepatology. 2004;40:656A. Abstract 1137. 2. Flink HJ, et al. Gut. 2005;54(11):1604-1609. 3. LauGKK et al. 56th AASLD;2005; San Francisco. Abstract 66086. 4. Fried MW, et al. Hepatology. 2005;42:268A. Abstract 182. 5. FlinkHJ, et al. Am J Gastroenterol. 2006;101(2):297-303. 6. Hadziyannis SJ, et al. J Hepatol. 2005;42(suppl 2):178. Abstract 49 Let’s Vote! Audience Response Question Which of the following factors may influence the selection of nucleos(t)ide analogue therapy: 2% 12% 2% 7% 78% A. B. C. D. E. Cost Genetic barrier to resistance Safety Potency All of the above Antiviral Agents: Safety, Tolerability, Cost, and Risk:Benefit LAM ADV Entecavir Telbivudine Tenofovir Dosing QD QD QD QD QD Tolerability Well tolerated Well tolerated; Watch serum Cr Well tolerated Well tolerated; Watch CPK Well tolerated; Watch serum Cr Pregnancy C C C B B 6500 8700 6000 6000 Approximate 2500 cost for 1 year Potency Moderate Modest High High High Resistance High Low High Low Moderate Dienstag JL. N Engl J Med. 2008;35(14):1486-500. HBeAg-Postive Chronic HBV: HBV DNA Suppression and HBe Seroconversion at 1 Year EASL Clinical Practice Guidelines. J Hepatol. 50(2009),doi:10.1016/j.jhep.2008.10.001; Dienstag JL. N Engl J Med. 2008;359(14):1486-1500. Higher Rates of Seroconversion With Longer Viral Suppression 30 27 22 26 21 % 20 10 3 5 2 1 0 48 weeks HBeAg loss HBsAg loss Heathcote J. EASL 2008. Abstract #1593 64 weeks HBeAb HBsAb HBeAg-Negative Chronic HBV: HBV DNA Suppression and ALT Normalization at 1 Year EASL Clinical Practice Guidelines. J Hepatol. 50(2009),doi:10.1016/j.jhep.2008.10.001; Dienstag JL.. N Engl J Med. 2008;35(14):1486-1500. Histologic improvement with long-term HBV DNA suppression with ETV Histologic Improvement* 96% 100 Proportion of patients (%) Proportion of patients (%) 100 80 Improvement in Ishak fibrosis score† 73% 60 40 20 41/56‡ 55/57 48 Weeks Long-term§ 0 88% 80 60 40 32% 20 18/56‡ 50/57 0 48 Weeks Long-term§ *≥2-point decrease in Knodell necroinflammatory score and no worsening of Knodell fibrosis score compared with baseline † ≥1-point decreasepatient had an inadequate Week 48 biopsy; ‡One § Median time of long-term biopsy: 280 weeks inadequate Week 48 biopsy; § Median time of long-term biopsy: 280 weeks Liaw Y-F et al. AASLD 2008; Poster #894 % With Disease Progression Clinical Outcomes by Treatment and Resistance Status 25 Placebo (n=215) YMDDm (n=209) (49%) Wild Type (n=221) 20 Placebo 21% YMDDm 13% WT 5% 15 10 5 0 0 6 12 18 24 Time After Randomization (Months) YF Liaw et al. N Engl J Med. 2004;351:1521-1531. 30 36 Incomplete Suppression of Virus Replication Leads to Resistance Dominant Strain Treatment Initiated Naturally Occurring Variants HBV Replication Drug Resistant Variant • Incomplete Suppression - Inadequate Potency/Drug Levels - Inadequate Adherence - Pre-Existing Resistance Variants Time Detection Level Fung SK & Lok ASF. Antivir Ther 2004; 9:1013–1026 Locarnini S, et al. Antivir Ther 2004; 9:679–693 Viral Suppression Reduces the Incidence of Resistance LAM in HBeAg-Positive Patients ADV in HBeAg-Negative Patients (Follow-up 29 months, n=159) (Follow-up 144 weeks, n=114) P < .001 between groups Yuen M et al. Hepatology. 2001; 34(4):785-791. Locarnini S et al. J. Hepatology . 2005;42(Suppl 2):17. Monitoring for Treatment Failure With Nucleos(t)ide Analogue Therapy • Primary nonresponse – Less than 1-log10 drop at week 12 • Partial virological response (detectable) – Week 24: LAM, LdT, ADV – Week 48: TDF, ETV • Virological breakthrough – Rise in serum HBV DNA (≥ 1.0 log10 IU/mL) above nadir Cumulative Incidence of HBV Resistance EASL Clinical Practice Guidelines. J Hepatol. 50(2009),doi:10.1016/j.jhep.2008.10.001; Dienstag JL. N Engl J Med. 2008;35(14):1486-1500 HBV Antiviral Therapy Cross-Resistance in Vitro LAM ETV LdT FTC ADV TDF ? ? HBV Resistance to Entecavir Affected by Previous Resistance to Lamivudine Cumulative Incidence (%) 100 Entecavir (naive): genotypic resistance Entecavir (lamivudine resistant): genotypic resistance 80 60 51 46 40 36 20 15 6 0 0.2 1 0.5 2 1.2 3 Year 1.2 4 Colonno RJ, et al. 42nd EASL;2007;Barcelona. Abstract 781; Lai CL, et al. Clin Infect Dis. 2003;36:687-696; Lok AS, et al. Gastroenterology. 2003;125:1714-1722; Tenney DJ, et al. 18th APASL; 2008:Seoul.. Abstract PL02. 1.2 5 TDF in Nucleos(t)ide-Experienced Patients: Undetectable* HBV DNA at Month 12 P = NS Undetectable* HBV DNA at Month 12 (%) P = NS 100 90 80 70 60 50 40 30 20 10 0 100 92 85 P =.001 92 90 73 30 All Patients n = 101 HBeAg Positive 85 HBeAg Negative 26 Wild-Type YMDD HBV Mutations 42 36 ADV-R No ADV-R 20 81 Virologic breakthrough not observed during follow-up period, independent of presence of ADV resistance at start of TDF *HBV DNA < 400 copies/mL (< 69 IU/mL) van Bömmel F, et al. 43rd EASL; 2008; Milan. Abstract 73. Case Presentation (cont’d) • Patient initiates treatment with TDF 300 mg/day – HBV DNA • 3 months: 120,000 IU/mL • 6 months: 785 IU/mL • 12 months: < 22 IU/mL • Serum Cr stable Keeffe EB, et al. Clin Gastroenterol Hepatol. 2007;5:890-897. HBV Therapy • HBV replication is closely linked to the lifetime risk of disease outcomes (HCC, ESLD) • New treatment paradigm = long-term control of HBV replication: – ↓ hepatic inflammation and fibrosis – ↓ risk of hepatic decompensation and/or HCC • First-line therapy – high potency/low resistance – – – – Peg-IFN Tenofovir Entecavir Combination antiviral therapy? Combination Therapy for Treatment of Chronic HBV Infection Marion Peters, MD Professor of Medicine Chief of Hepatology Research University of California, San Francisco San Francisco, California Case Presentation • A 45-year-old man was admitted with fatigue, malaise, and abdominal swelling in June 2003 • He was born in Greece, came to United States at age 14 • His brother had a liver transplant for HBV in 1998 • Examination reveals jaundice, ascites, no muscle wasting, spider nevi Case Presentation: HBV Laboratory • Bilirubin 3.7, AST 129, ALT 106, albumin 2.4, PT 1.7, ammonia 51, creatinine 0.9 • MELD (model for end-stage liver disease) score, 19 • HBsAg and HBeAg positive • HBV DNA 1.7 billion copies per mL • AFP 741 µg/L • Paracentesis WCC 183, albumin <1 Let’s Vote! Audience Response Question How would you treat his HBV? 10% A. Pegylated interferon (Peg-IFN) for 48 weeks 5% B. Lamivudine (LAM) 100 mg per day 1% C. Adefovir (ADV) 10 mg per day 23% D. Entecavir (ETV) 0.5 mg per day 3% E. Telbivudine (LdT) 600 mg 22% F. Tenofovir (TDF) 300 mg per day 37% G. Combination LAM + TDF Case Presentation: Treatment • June 2003 started LAM 100 mg daily - Well tolerated - Patient has improvement in well-being • Listed for liver transplantation • Ultrasound: cirrhotic liver, no masses • CT, quadruple phase: no masses Case Presentation: Laboratory Findings LAM Date AST Bili Albumin AFP HBV DNA 6-03 160 3.7 2.5 741 1,700,000,000 11- 03 59 0.9 3.1 14 2,000,000 copies/mL Case Presentation: Laboratory Findings (cont’d) LAM Date AST Bili Albumin AFP HBV DNA 6-03 160 3.7 2.5 741 1,700,000,000 11- 03 59 0.9 3.1 14 2,000,000 2-04 74 1.3 2.9 193 2,500,000,000 copies/mL Let’s Vote! Audience Response Question What has occurred? 5% A. LAM nonresponse 87% B. LAM resistance 8% C. Noncompliance HBeAg-Positive Patients (N = 159) Median Follow-up: 29.6 Months 100 Patients With YMDD Variants (%) Patients With Resistance (%) HBV DNA at Month 6 of LAM Predicts Later Risk of Resistance 80 64 60 40 20 32 8 13 12 23 41 118 0 n= ≤2 ≤3 ≤4 >4 HBV DNA at 6 Months (log10 copies/mL) Yuen ME et al. Hepatology. 2001;34:785-791. Case Presentation: HBV Status • HBV genotype A, HBeAg positive • Polymerase mutations - L180M, +M204V - No precore mutations detected - No ADV mutations detected • HIV negative • Hepatitis D virus negative Let’s Vote! Audience Response Question How would you treat his HBV? 3% A. Switch to ADV 10 mg per day 7% B. Switch to ETV 0.5 mg per day 15% C. Switch to TDF 300 mg per day 19% D. Add ADV 10 mg per day 8% E. Add ETV 0.5 mg per day 47% F. Add TDF 300 mg per day Case Presentation: Laboratory Findings (cont’d) LAM Add ADV Date AST Bili Albumin AFP 6-03 160 3.7 2.5 741 1,700,000,000 11- 03 59 0.9 3.1 14 2,000,000 2-04 74 1.3 2.9 193 2,500,000,000 HBV DNA copies/mL Case Presentation: Laboratory Findings (cont’d) LAM Add ADV Date AST Bili Albumin AFP 6-03 160 3.7 2.5 741 1,700,000,000 11- 03 59 0.9 3.1 14 2,000,000 2-04 74 1.3 2.9 193 2,500,000,000 5-04 69 1.5 3.0 169 1,600,000,000 HBV DNA copies/mL Let’s Vote! Audience Response Question What has occurred? 10% A. ADV resistance 28% B. ADV primary nonresponse 54% C. ADV suboptimal response 4% D. Worsening liver failure 4% E. Noncompliance Nonresponse, Suboptimal Response, and Virologic Breakthrough Change in HBV DNA (log10 IU/mL) 1.0 Antiviral Drug Primary nonresponse 0 Virologic breakthrough -1.0 Suboptimal response -2.0 -3.0 Nadir -4.0 0 6 12 Months Lok AS et al. Hepatology. 2007;45:507-539. 1 log 18 HBV DNA at Week 48 of ADV Predicts Risk of Resistance at Week 144 N = 114 Patients, Primarily HBeAg Negative1 Resistance (%) 100 100 N = 124 Patients, HBeAg Negative2 80 80 67 60 60 40 40 49 26 20 20 6 4 0 0 <3 3-6 >6 <3 HBV DNA at Week 48 (log10copies/mL) 1. Locarnini S et al. 40th EASL; 2005; Paris. Abstract 36. 2. Hadziyannis SJ et al. Gastroenterology. 2006;131:1743-1751. >3 Let’s Vote! Audience Response Question What would you do? 2% A. Continue ADV 17% B. Add TDF 300 mg 9% C. Change to TDF and ADV 34% D. Change to TDF and LAM or emtricitabine (FTC) 39% E. Change to TDF and ETV Case Presentation: Laboratory Findings (cont’d) Date AST Bili LAM 6-03 160 3.7 2.5 741 1,700,000,000 Add ADV 11- 03 59 0.9 3.1 14 2,000,000 2-04 74 1.3 2.9 193 2,500,000,000 5-04 69 1.5 3.0 169 1,600,000,000 8-04 68 1.8 3.4 42 78,000,000 11-04 67 1.0 3.7 16.2 97,000 5-06 52 1.1 4.0 8 2,590 5-07 28 1.0 4.4 2.9 Switch to TDF + LAM Albumin AFP HBV DNA undetectable <5 copies/mL Why Consider Combination Therapy? • Sequential monotherapy with nucleos(t)ide analogs has led to HBV resistance • There may be special populations in whom combination is recommended: - Cirrhotics in whom development of resistance may have irreversible severe consequences - HIV/HBV coinfected • Changing to another nucloes(t)ide after failure increases chance of poor or nonresponse and of resistance to next drug, eg, ETV • Multidrug-resistant mutants described after sequential monotherapy1,2 • Resistance has been low with combination therapy 1. Yim HJ et al. Hepatology. 2006:43:S173-181. 2. Shaw T et al. 58th AASLD; 2007; Boston. Abstract 986. Combination Therapy • Peg-IFN and LAM showed more HBV DNA suppression while patients on therapy but suppression lost after end of therapy; no increased HBeAg seroconversion • ADV and LAM/FTC: less resistance but no increase in efficacy Lampertico P et al. Gastroenterology. 2007;133(5):1445-1451. Patients (%) Patients (%) PEG-IFN alfa-2a +/- LAM for HBeAg+ CHB Week 48 (End of therapy) *P<.05 vs lamivudine **P<.01 vs lamivudine ***P<.001 vs lamivudine Week 72 (24 weeks off-therapy) Lau GK et al. N Engl J Med 2005; 352(26):2682-2695. ADV vs ADV + LAM for HBeAgLAM-Resistant Patients • Multicenter cohort study; retrospective/prospective ‒ Mean follow-up: 33 months Undetectable HBV DNA* (%) ADV + LAM (n = 285) ADV (n = 303) 100 Patients (%) Year 3 Cumulative ADV Resistance 100 80 80 60 60 P = NS 40 40 20 20 0 0 0 6 12 18 Month *< 1000 copies/mL. 24 30 36 P < .001 16 0 ADV (n = 303) ADV + LAM (n = 285) Lampertico P et al. Gastroenterology. 2007;133(5):1445-1451. Lampertico P et al. 57th AASLD; 2006; Boston. Abstract LB5. CCO ADV Resistance Not Observed With LAM Combination Therapy Incidence of Resistance (%) ADV monotherapy (Study 438: naive patients) 60 ADV+ LAM (Studies 435 and 460i: LAM resistance*; Study 435: pre- and post-OLT; Study 460i: HIV/HBV) 40 30 20 19 11 0 0 0 Year 1 3 0 0 Year 2 Year 3 0 Year 4 Year 5 *Two patients enrolled in Study 435 initially received combination therapy with adefovir + lamivudine and subsequently selected adefovir resistance mutation N236T. However, they had switched to adefovir monotherapy at a time when adefovir resistance mutation was detected. Lee YS,et al. Hepatology. 2006;43:1385-1391. Lampertico P et al. 57th AASLD; 2006; Boston. Abstract LB5. Schiff E et al. Liver Transpl. 2007;13:349-360. Hepsera [package insert]. Managing Responses in the Treatment of CHB Week 12 Assess for primary nonresponse Week 24 Assess early predictors of efficacy Complete response HBV DNA negative by PCR Partial response HBV DNA 60 to < 2000 IU/mL Inadequate response HBV DNA ≥ 2000 IU/mL Continue Monitor every 6 months Add another drug without cross-resistance or continue Monitor every 3 months Add/switch to more potent drug Monitor every 3 months Adapted from Keeffe E et al. Clin Gastroenterol Hepatol. 2007;5:890-897. Combination Therapy • Combination therapy has reduced the emergence of resistance • This may lead to better long-term outcomes • At present no FDA-approved indication for use of combination therapy in patients with chronic HBV infection and no synergy in HBV DNA decline noted • Use in cirrhotic patients especially those with preexisting YMDD mutations and in HIV HBV patients appears warranted Jacobson IM. J Hepatol. 2008;48:687-691; CDC Guidelines for HIV patients. 2008.