Biochemistry 2/e - Garrett & Grisham
Chapter 15
Enzyme Specificity and
Regulation
to accompany
Biochemistry, 2/e
by
Reginald Garrett and Charles Grisham
All rights reserved. Requests for permission to make copies of any part of the work
should be mailed to: Permissions Department, Harcourt Brace & Company,
6277
Sea Harbor Drive, Orlando, Florida 32887-6777
Biochemistry 2/e - Garrett & Grisham
Outline
•
•
•
•
•
15.1 Specificity from Molecular Recognition
15.2 Controls over Enzymatic Activity
15.3 Allosteric Regulation of Enzyme Activity
15.4 Allosteric Model
15.5 Glycogen Phosphorylase
• SPECIAL FOCUS: Hemoglobin and Myoglobin
Biochemistry 2/e - Garrett & Grisham
15.1 Specificity
The Result of Molecular Recognition
• Substrate (small) binds to enzyme
(large) via weak forces - what are they?
– H-bonds, van der Waals, ionic
– sometimes hydrophobic interactions
• Understand the lock-and-key and
induced-fit models
• Relate induced-fit to transition states
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
15.2 Controls over Enzyme
Activity
•
•
•
•
•
•
Six points:
Rate slows as product accumulates
Rate depends on substrate availability
Genetic controls - induction and repression
Enzymes can be modified covalently
Allosteric effectors may be important
Zymogens, isozymes and modulator
proteins may play a role
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
15.3 Allosteric Regulation
•
•
•
•
Action at "another site"
Enzymes situated at key steps in
metabolic pathways are modulated by
allosteric effectors
These effectors are usually produced
elsewhere in the pathway
Effectors may be feed-forward activators
or feedback inhibitors
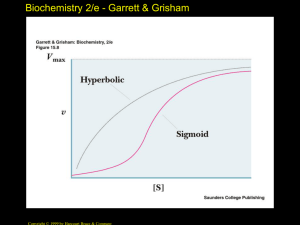
Kinetics are sigmoid ("S-shaped")
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Models for Allosteric Behavior
• Monod, Wyman, Changeux (MWC)
Model: allosteric proteins can exist in
two states: R (relaxed) and T (taut)
• In this model, all the subunits of an
oligomer must be in the same state
• T state predominates in the absence of
substrate S
• S binds much tighter to R than to T
Biochemistry 2/e - Garrett & Grisham
More about MWC
• Cooperativity is achieved because S
binding increases the population of R,
which increases the sites available to S
• Ligands such as S are positive
homotropic effectors
• Molecules that influence the binding of
something other than themselves are
heterotropic effectors
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Glycogen Phosphorylase
•
•
•
•
Allosteric Regulation and Covalent
Modification
GP cleaves glucose units from
nonreducing ends of glycogen
A phosphorolysis reaction
Muscle GP is a dimer of identical
subunits, each with PLP covalently
linked
There is an allosteric effector site at the
subunit interface
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Glycogen Phosphorylase
•
•
•
•
Allosteric Regulation and Covalent
Modification
Pi is a positive homotropic effector
ATP is a feedback inhibitor, and a
negative heterotropic effector
Glucose-6-P is a negative heterotropic
effector (i.e., an inhibitor)
AMP is a positive heterotrophic effector
(i.e., an activator)
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Regulation of GP by Covalent
Modification
• In 1956, Edwin Krebs and Edmond
Fischer showed that a ‘converting
enzyme’ could convert phosphorylase b
to phosphorylase a
• Three years later, Krebs and Fischer
show that this conversion involves
covalent phosphorylation
• This phosphorylation is mediated by an
enzyme cascade (Figure 15.19)
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
cAMP is a Second Messenger
• Cyclic AMP is the intracellular agent of
extracellular hormones - thus a ‘second
messenger’
• Hormone binding stimulates a GTPbinding protein (G protein), releasing
G(GTP)
• Binding of G(GTP) stimulates adenylyl
cyclase to make cAMP
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Hemoglobin
•
•
•
•
•
A classic example of allostery
Hemoglobin and myoglobin are oxygen
transport and storage proteins
Compare the oxygen binding curves for
hemoglobin and myoglobin
Myoglobin is monomeric; hemoglobin is
tetrameric
Mb: 153 aa, 17,200 MW
Hb: two alphas of 141 residues, 2 betas of 146
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Hemoglobin Function
Hb must bind oxygen in lungs and
release it in capillaries
• When a first oxygen binds to Fe in
heme of Hb, the heme Fe is drawn into
the plane of the porphyrin ring
• This initiates a series of conformational
changes that are transmitted to adjacent
subunits
Biochemistry 2/e - Garrett & Grisham
Hemoglobin Function
Hb must bind oxygen in lungs and
release it in capillaries
• Adjacent subunits' affinity for oxygen
increases
• This is called positive cooperativity
Biochemistry 2/e - Garrett & Grisham
Myoglobin Structure
•
•
•
•
•
Mb is a monomeric heme protein
Mb polypeptide "cradles" the heme group
Fe in Mb is Fe2+ - ferrous iron - the form
that binds oxygen
Oxidation of Fe yields 3+ charge - ferric
iron -metmyoglobin does not bind oxygen
Oxygen binds as the sixth ligand to Fe
See Figure 15.26 and discussion of CO
binding
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
The Conformation Change
•
•
•
•
•
•
•
The secret of Mb and Hb!
Oxygen binding changes the Mb conformation
Without oxygen bound, Fe is out of heme plane
Oxygen binding pulls the Fe into the heme plane
Fe pulls its His F8 ligand along with it
The F helix moves when oxygen binds
Total movement of Fe is 0.029 nm - 0.29 A
This change means little to Mb, but lots to Hb!
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Binding of Oxygen by Hb
The Physiological Significance
• Hb must be able to bind oxygen in the lungs
• Hb must be able to release oxygen in
capillaries
• If Hb behaved like Mb, very little oxygen
would be released in capillaries - see Figure
15.22!
• The sigmoid, cooperative oxygen binding
curve of Hb makes this possible!
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Oxygen Binding by Hb
•
•
•
•
A Quaternary Structure Change
When deoxy-Hb crystals are exposed to
oxygen, they shatter! Evidence of a
structural change!
One alpha-beta pair moves relative to the
other by 15 degrees upon oxygen binding
This massive change is induced by
movement of Fe by 0.039 nm when oxygen
binds
See Figure 15.32
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
The Bohr Effect
•
•
•
•
Competition between oxygen and H+
Discovered by Christian Bohr
Binding of protons diminishes oxygen binding
Binding of oxygen diminishes proton binding
Important physiological significance
• See Figure 15.34
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Bohr Effect II
Carbon dioxide diminishes oxygen
binding
• Hydration of CO2 in tissues and
extremities leads to proton production
• These protons are taken up by Hb as
oxygen dissociates
• The reverse occurs in the lungs
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
2,3-Bisphosphoglycerate
An Allosteric Effector of Hemoglobin
• In the absence of 2,3-BPG, oxygen
binding to Hb follows a rectangular
hyperbola!
• The sigmoid binding curve is only
observed in the presence of 2,3-BPG
• Since 2,3-BPG binds at a site distant from
the Fe where oxygen binds, it is called an
allosteric effector
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
2,3-BPG and Hb
The "inside" story......
• Where does 2,3-BPG bind?
– "Inside"
– in the central cavity
• What is special about 2,3-BPG?
– Negative charges interact with 2 Lys, 4 His, 2
N-termini
• Fetal Hb - lower affinity for 2,3-BPG, higher
affinity for oxygen, so it can get oxygen from
mother
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham