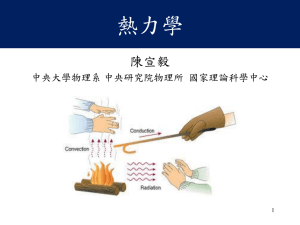
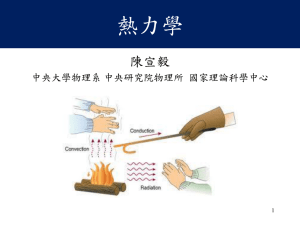
熱力學(陳宣毅)
advertisement

熱力學 陳宣毅 中央大學物理系 中央研究院物理所 1 Outline 大綱 • Prelude, 序幕 • What is heat? 什麼是熱? • Thermodynamics and time’s arrow. 熱力學與時間之箭 • Thermodynamics and atomic hypothesis. 熱力學與原子假說 • Thermodynamics and 21th century science. 熱力學與二十一 世紀的科學 2 References 參考資料 • 網際網路……(copy and paste!) • Wikipedia (most of the time very reliable), 維基百科 • The 2nd law, by Atkins. (good book for fun) 3 Prelude 序幕 4 What is NOT thermodynamics? 什麼不是熱力學? 1. pV = nRT 2. Q = mcT 3. L = aT http://en.wikipedia.org/wiki/Thermodynamics • Thermodynamics is closer to answering the following question: What makes 1-3 true? 熱力學比較接近: 回答為何1-3 為真 5 What is thermodynamics? 什麼是熱力學? • Physics of systems with many degrees of freedom, 研究由許多分子構成的系統(巨觀系統) • Built upon a few observations (laws, no proof), 建構在幾個對自然現象的觀察上 (無法証明基本假設 為真) • Thermodynamic laws are independent of fundamental interactions between constituent particles, 熱力學定律不是建立於分子間的基本交互作用上 • Results derived from these laws provides absolute constraint on the behavior of systems with many degrees of freedom, 熱立學對巨觀系統的可能行為給出了絕對的規範 6 Rules of the game 關於熱力學的遊戲規則 • Rule number 1: basic ideas should come from experimental observations. 基本觀念必須建立在實驗結果上 • Rule number 2: a new idea is not meaningful if one cannot link it to experiments. 與實驗無關的理論沒有熱力學上的意義 • Rule number 4: if an experimental result contradicts with an idea, then one should check if the contradiction is a result of the conceptual mistakes of the ideas or is an artifact of the experiment. 實驗結果若與理論不符,必須檢查究竟是實驗或理論出錯 7 In thermodynamics, there are 4 laws 熱力學有四個定律 • Zeroth law: equilibrium and temperature 熱平衡與溫度 • 1st law: what is heat? 熱是什麼? • 2nd law: time’s arrow 時間的方向 • 3rd law: low temperature limit 低溫的極限 8 Zeroth and 1st laws: equilibrium, temperature, and heat 第零與第一定律: 平衡,溫度,與熱 9 Zeroth law : temperature and equilibrium 第零定律: 溫度與熱平衡 A B C From experimental observations: If A is in thermal equilibrium with B, at the same time B is in thermal equilibrium with C, then A is in thermal equilibrium with C. 若 A與B達成熱平衡,且同時B與C達成熱平衡,則A與C也處於熱平衡的 狀態。 TA=TB, TB=TC TA=TC. The concept of temperature is physically meaningful. 溫度為有意義的物理量 10 1st law: What is heat? 什麼是熱? • From Wikipedia, the free encyclopedia: In physics, heat, symbolized by Q, is defined as a form of energy whose absorption raises the temperature of a body, not existing in the transition state, and abstraction of which from the same body lowers its temperature. Generally, heat is a form of energy transfer, sometimes called thermal energy, associated with the different motions of atoms, molecules and other particles that comprise matter when it is hot and when it is cold. Heat: a form of energy or a form of energy transfer? 熱是能量的一種形式還是能量轉移的方式? 11 What is heat, really? 到底什麼是熱? • Heat is a form of energy. 熱是一種能量(很抽象) (now it is meaningful to say: “absorb heat” or “release heat”. ) • Heat goes spontaneously from high T region to low T region. 熱能會自發地由高溫的區域流向低溫的區域 • A definition in terms of mathematics is needed! 如何以數學的形式定義熱? • Energy is …… E = mv2/2 + V(x) Heat is …… (can you find Q=……?) We will find that it is only possible to write: “Q=……”, (heat is a form of energy transfer) 我們只能定義熱能的改變量(熱是一種能量轉移的方式)12 Heat is a form of energy: Joule’s experiment 熱是一種能量: 焦耳的實驗 Mgh = W = Q = mcT James Joule (1818-1889) Mechanical energy can turn into heat, then raise temperature of the system. 力學能可以轉變為熱能,提高系統的溫度 13 Heat and the 1st law of thermodynamics 熱與熱力學第一定律 • Mechanics: W = E, (E: energy of the system) • In the presence of friction W = E Q, (Q: heat absorbed by the system) • In general W = E Q 1st law of thermodynamics (heat comes from friction, heat conduction, etc) 外界對系統作的功等於系統內能增加量減去系 統所吸收的熱 14 How does “heat energy” look like? 如何將熱能“圖像化”? •Atoms in the lattice are dragged by the tip. 晶體表面原子被針尖拖曳 •The lattice begin to vibrate randomly. 晶體開始不規則振動 •Work done by external force is transformed into this random vibration. 針尖作的功轉變為晶體的不規則運動 •Heat = irregular motion at molecular scale. 熱 = 分子尺度不規則運動的能量 15 http://www.nccr-nano.org/nccr/media/nanonews/nanonews_05/highlights/highlight_13 The implications of 1st law 第一定律的意義 E = W + Q 1. Heat is a form of energy. 熱是能量的一種形式 2. Only energy-conserving process can occur. 只有能量守恆的過程才可能發生 3. Heat can be used to perform work. (heat engine) 熱可以用來作功 16 2nd law: thermodynamics and time’s arrow 第二定律: 熱力學與時間之箭 17 Heat engine 熱機(引擎) TH Q1 W • Heat engine:: extracts heat Q1 from reservoir TH, releases heat Q2 to reservoir TC, performs work W = Q1 Q2. 從高溫處吸熱,將熱轉為功,多餘的 熱傳至低溫處。 • Efficiency = W/Q1 = 1 – Q2/Q1 Q2 TC • Can eventually efficiency 1? 效能是否可以趨近100%? 18 Efficiency of a heat engine 熱機的效能 TH Nicolas Léonard Sadi Carnot (1796-1832), mathematician and one of the pioneers of thermodynamics. Q1 W Q2 • Efficiency = W/Q1 = 1 – Q2/Q1 TC • Carnot: efficiency 1 TC/TH • There is no way to transform heat 19 100% into work! Carnot’s engine of highest possible efficiency 卡諾的最佳效能熱機 http://www.grc.nasa.gov/WWW/K-12/airplane/carnot.html 20 Kelvin statement of the 2nd law of thermodynamics 克爾文對熱力學第二定律的敘述 TH Q1 W Q2=0 TC Lord Kelvin(1824-1907): a transformation whose only final result is to convert heat, extracted from a source at constant temperature, into work, is impossible. 熱無法完全轉變為功 21 Clausius statement of the 2nd law of thermodynamics 克勞修斯對熱力學第二定律的敘述 TH Q TC Rudolf Clausius (1822-1888): heat cannot of itself pass from a colder to a hotter body. 熱不會自動由低溫處流向高溫處 22 The meaning of 2nd law 第二定律的意義 • Energy is conserved in all processes, but not all energy conserving processes can happen! 所有發生的過程都滿足能量守恆,但不是所有能量守恆的過程都會發生。 • 100% heat work (process A) cannot occur, but 100% work heat (time reversal of A) can occur. • 2nd law refers to macroscopic processes only. 第二定律只適用於巨觀系統 • Some macroscopic processes are not reversible! (Time’s arrow exists.) 有一些巨觀過程是不可逆的 (時間有一定的方向) 23 Question: relation between 2nd law and molecular motion 第二定律與分子運動的關係 • 2nd law refers to macroscopic processes only. Why? 為何第二定律只適用於巨觀系統? • 100% heat work (process A) cannot occur, but 100% work heat (time reversal of A) can occur How do motion of molecules give us 2nd law? 分子尺度的運動如何造成巨觀的第二定律? 24 Microscopic reversibility 微觀物理的可逆性 • F = m a; F = F(x); a = d2x/dt2 • Time reversal: t t, x x • v v • F F, a a • F = m a is valid under time reversal. There is no time’s arrow in microscopic physics. 牛頓運動定律(基本力學定律)在時 間反演下仍然成立 25 Microscopic reversible, macroscopic irreversible? 微觀物理定律可逆,巨觀物理定律不可逆? TH Q Raindrop splash and displacement of soil particles. (USDA Natural Resources Conservation Service) TC 26 Microscopic illustration of 2nd law 從分子運動看第二定律 • http://comp.uark.edu/~jgeabana/mol_dyn/KinThI.html • Reverse the movie, you don’t see original lattice…… 倒放電影,無法回到原始的晶格 • A small change in the initial condition (due to precision of the computer) is amplified. 由於電腦精確度的限制,倒放電影的初始態有微小的誤 差,此誤差被放大所以無法回到原始的晶格。 • The initial condition (lattice) is an unlikely arrangement, therefore you never see it again! 原始的晶格是極難達成的排列,所以你無法在倒放的電 影看見它 27 How to describe irreversibility quantitatively? 如何定量描述巨觀不可逆現象? Example: mixing sucrose with water 將糖溶於水 Time’s arrow: maximize “number of ways” to arrange the molecules. 時間之箭指向增加分子排列方式的方向 28 “Number of ways” to arrange the molecules “分子排列的方式” Two ways to describe the physical state of a system. 兩種描述一系統物理狀態的方法 1. Mechanical state (microscopic state): momentum and position of all particles: (r1, r2,… ,rN; p1, p2,… ,pN), (6N variables). 力學狀態: 描述所有粒子的位置與動量(速度),共需6N個數字 2. Thermodynamic state (macroscopic state): E,T,V,p,N,…. (much less than 6N variables) 熱力學狀態: 描述內能、溫度、體積、壓力、粒子數等,需要用 到遠小於6N個數字 A macroscopic state corresponds to many microscopic states. W(A): number of microscopic states for a macroscopic state A(T,V,N,…). 一個熱力學狀態對應於許多力學狀態, W(A)>>1. 29 Boltzmann’s idea Ludwig Eduard Boltzmann (1844-1906) S: entropy 熵 S = k lnW 30 Clausius and entropy 克勞修斯與熵 • In mechanics V/x = F for conservative forces V/x < F for nonconservative forces We define potential energy V (only depend on x). • Clausius found (before Boltzmann): TS = Q for reversible processes TS > Q for irreversible processes We define entropy S (only depends on macroscopic state of the system). 熵是狀態函數 31 “S” statement of 2nd law 第二定律的“S”敘述 S = klnW, TS Q • Entropy of a thermally isolated system cannot decreases. 獨立系統的熵必須增加或不變 • Microscopically, time’s arrow: maximize “number of ways” to arrange the molecules. 微觀: 時間之箭指向增加分子排列方式的方向 • Macroscopically, time’s arrow in the universe points toward where entropy of the universe increases. 巨觀:時間之箭指向熵增加的方向 32 3rd law: low temperature limit 第三定律: 低溫極限 Boltzmann: S = k lnW absolute magnitude of entropy 波茲曼: 以分子排列方式定義熵的(絕對)大小 Clausius: S = Q/T (reversible processes) entropy difference between two states 克勞修斯: 從可逆過程定出熵在兩不同熱力學狀態的差 3rd law: 1. Provides a link between two definitions of entropy 連結兩種熵的定義 2. States the experimental low temperature limit. 描述實驗低溫的極限 33 3rd law: entropy at zero temperature • Lower temperature release heat from the system • Lowest energy = T 0 • Lowest energy microscopic state: there is only one such state! (sometimes not one, but just a few) • Nernst’s theorem: No finite sequence of cyclic processes can succeed in cooling a body to absolute zero temperature. S(T=0) = k ln(1) = 0. Walther Hermann Nernst (1864-1941) 34 Thermodynamics and atomic hypothesis 35 Atomic hypothesis • Feynman lectures on physics, Vol 1, Ch.1: If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures…… I believe it is the atomic hypothesis…… all things are made of atoms – little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. 36 When you think about atomic scale… • Looking for basic physical laws at small scale quantum physics, elementary particles. • Connecting macroscopic phenomena with dynamics at atomic scale thermodynamics and heat . 37 The importance of atomic hypothesis From http://physicalworld.org/restless_universe/html/ru_bolt.html Boltzmann's contribution was vital, but had a tragic outcome. Towards the end of the nineteenth century several puzzling facts (which eventually led to quantum theory), triggered a reaction against 'materialist' science, and some people even questioned whether atoms exist. Boltzmann, whose work was based on the concept of atoms, found himself cast as their chief defender and the debates became increasingly bitter. Always prone to bouts of depression, Boltzmann came to believe that his life's work had been rejected by the scientific community, although this was far from being true. In 1906, he committed suicide. If despair over rejection, or frustration over being unable to prove his point, were contributing factors the irony would be great indeed. Soon after Boltzmann's death, clinching evidence was found for atoms, and few would ever doubt their existence again 38 Brownian Motion and thermodynamics 400x, plastic spheres, each 913 nm in diameter http://physics.ius.edu/~kyle/K/Brownian/Brownian.html 39 Robert Brown and Brownian motion Brown (1827): observed irregular movement of pollens in water under microscope. [First observation of “Brownian motion”: S. Gray, Phil. Trans. 19, 280, (1696). ] Robert Brown Major contribution of Brown: made sure non-organic particles also have Brownian motion, confirmed that Brownian motion is not a manifestation of life. 40 Early theories of Brownian motion • From energy of light in the microscope? • Surface tension effect? • (1889) temperature difference between the solution and environment? • Puzzle: average speed of Brownian particles V t1/2 ? • Why were scientists unable to explain Brownian motion? 41 Einstein, Brownian motion, and atomic hypothesis The Miracle year: Albert Einstein published 4 papers in the Annalen der Physik in 1905. – Photoelectric effect – Brownian motion – Special theory of relativity Which topic is his PhD thesis? Albert Einstein, 1905 42 Einstein's theory of Brownian motion 沒有外場的布朗運動: v ~ t-1/2 即: 運動距離 d(t) ~ t1/2 愛因斯坦: 1. 花粉在溶液中不斷因水分子的碰撞而改變運動方向。 2. 兩次碰撞(即走三步)後的位移 x = x1 + x2 + x3 3. 平均位移 <x> = <x1> + <x2> + <x3> = 0 因為兩次碰撞間朝 任何方向移動的機率都相同。 4. 但是 <x2> = <x12> + <x22> + <x32> + 2 <x1x2> + 2 <x2x3> + 2 <x3x1> 5. <x1x2> = <x1><x2> = 0, <x2x3> = 0, <x3x1> = 0. 因每一步所走 的方向與其他步無關(獨立事件)。 6. 故<x2> = <x12> + <x22> + <x32> = 3 <x12> 7. 走N步: <x2> = N <x12> ~ t 8. 平均移動的距離 d = (<x2>)1/2 ~ t1/2 , 平均速率 ~ t 1/2 43 From Brownian motion to Avogadro number 1. 花粉在水中的布朗運動: d2 = 6Dt, D:擴 散係數 2. 花粉在水中的運動受水的黏滯力: f = gv, g:阻泥係數 3. [D] = L2/T; [g]=[f]/[v]=M/T 4. [Dg] = ML2/T2 = [E] 5. 愛因斯坦: (i) 水分子撞花粉靜止的花粉因而獲 得能量行布朗運動。 (ii) 花粉運動受黏滯力而將能量傳回水 分子。 (iii) 要達成熱平衡需要Dg = kT = RT/NA (iv)亞佛加厥數NA=RT/Dg 實驗:量T, g, D,得NA Perrin: NA = 7×1023 Jean B. Perrin Nobel Prize for physics: 1926 44 Einstein relation • Drag force: f = gv • Diffusion due to random walk: d2 = 6Dt • To reach equilibrium: Dg = kT • Random collisions (random walk) are related to the dissipation of kinetic energy to solvent molecules. 45 Atomic picture of thermal equilibrium Big ball gets kinetic energy from small balls from random collisions. Small balls gets kinetic energy from big ball from viscous drag (turns kinetic energy of the big ball into heat). Equilibrium: energy from big ball to small balls = energy from small balls to big ball http://www.unmuseum.org/einstein.htm Equilibrium kinetic energy of the big ball = equilibrium kinetic energy of a small ball = (3/2)kT. 46 Thermodynamics: from 19th to 21th century science 47 Heat conduction (19th century science) • http://www.gcse.com/energy/conduction.htm 48 Electric conduction (20th century science) http://people.deas.harvard.edu/~jones/es154/lectures/lecture_2/drude_model/drude _model.html J=sE Question: s as T ? as T ? 49 Motor proteins 50 Nanomachines: Brownian motor (21th century science) Motor: +2 ATP: -2 1. Motor+2+ATP-2 MotorATP 2. MotorATP Motor+2 + ADP+P- 51 Physics of Brownian motors 1. Symmetry 2. Thermal equilibrium 3. Time scale: on-off time b2 > Dt > a2 52 A molecular motor at work ATPase: 製造ATP的蛋白質 H. Noji, R. Yasuda, M. Yoshida, K. Kinoshita Jr, Nature, 386, 299 (1997) http://www.k2.phys.waseda.ac.jp/F1movies/F1long.htm 53 “Nano-robotics”: 大腸菌 http://en.wikipedia.org/wiki/Escherichia_coli 54 Molecular motors drive E. coli swim 55 Rotating flagella make E. coli move Our dream: Make machines that move like them! http://www.rowland.harvard.edu/labs/bacteria/showmovie.php?mov=fluo_cell_near 56 Thermodynamics and the origin of the universe • Unsolved problems in physics: Arrow of time : Why did the universe have such low entropy in the past, resulting in the distinction between past and future and the second law of thermodynamics? http://en.wikipedia.org/wiki/Entropy_%28arrow_of_time%29 57 Epilogue: “more is different” • Heat is a form of energy. • Not all energy conserving processes occur (time’s arrow). • Biological active motion is supported by energy source (we are not in thermal equilibrium). • “fundamental physical laws” may “change”, but thermodynamic laws are there. 58