PhotoElectric effect
advertisement

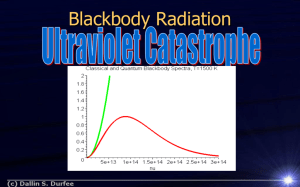
PHOTOELECTRIC EFFECT 13.1.1-13.1.4 Reading Recommendation: Pages 910-918 PHOTOELECTRIC EFFECT A phenomenon in which a metal is struck by incoming light, which causes electrons from the metal to be emitted from the metal’s surface. Examples where this can be observed: Light shining on a negatively-charged electroscope causes it to be discharged Light shining on a neutral electroscope causes it to become charged (positively) PHOTONS…AND WHAT EINSTEIN THOUGHT OF THEM What is a photon? It is a “packet” of light “Quantized” light Light acting as if it were a particle, not a wave, that is carrying its energy. Wait…Quantized? What kind of word is that? It means that there is a distinct, discrete amount of energy being carried by a “particle” of light, dependent on the qualities of the light. Waves carry energy continuously, and in varying amounts/intensities depending on the position within the wave at which you look…continuous ≠ discrete EINSTEIN’S LOGIC… Radiant light is emitted from objects that have a distinct amount of thermal energy (i.e. those objects that are at a measurable temperature emit radiant energy, the magnitude of which is dependent on the temperature) If the kinetic energy of the atoms is discrete And if the kinetic energy determines the temperature… Shouldn’t the radiant energy being emitted also be discrete? AND WHAT IF IT’S NOT? IF the radiant energy being emitted from the hotter object is NOT discrete, then there is no way to show that energy is conserved during the thermal energy transfer from hot object to cooler object/surroundings However, it has been shown time and again that energy IS conserved. So Einstein is probably right. (and earned the 1921 Nobel Prize in Physics as a result) DEMOS OF THIS EFFECT: Demo 1: https://www.youtube.com/watch?v=WO38qVDGgqw Demo 2: Phosphorescent strip (done in person in class ) Watch this video describing the photoelectric effect Demo 3 (watch at home): https://www.youtube.com/watch?v=kcSYV8bJox8 ELECTRONVOLT Reminder: An electronvolt (eV) is a measure of the amount of energy gained by an electron as it travels through a potential difference of exactly 1 V 1 eV = 1.6 x 10-19 J We will be using electronvolts as our energy unit throughout this topic! BLACK BODY RADIATION Objects with measurable temperatures will radiate heat in the form of radiant energy Depending on the temperature, different wavelengths of light will be emitted WIEN DISPLACEMENT LAW Relates the wavelength of light with the temperature of the Black Body: max T 2.9 10 m K 3 For example…determine the surface temperature of an object that predominantly emits in the visible spectrum: STEFAN-BOLTZMANN LAW Relates the total energy being emitted by the blackbody to its temperature: CLASSICAL THEORY RESULT: Combining the Wien law and the StefanBoltzmann law, it was shown, classically, that 1 I 4 What happens as the wavelength gets shorter? Ultraviolet Catastrophe! MAX PLANCK Assumptions made to explain why the ultraviolet catastrophe doesn’t actually happen Thermal Oscillators (the atoms emitting the radiation) have a specific, discrete amount of energy Unlike blackbody radiation, the energy was not emitted in a broad, continuous spectrum Energy was emitted in packets of energy, equivalent to the amount of energy contained by the atoms. PLANCK’S HYPOTHESIS Frequency of the emitted radiation is proportional to the energy the radiation carries away from the thermal oscillator. Planck’s constant (an experimental value!) = 6.63 x 10-34 J·s = 4.14 x 10-15 eV·s QUANTUM OF ENERGY The term given to the quantity hf Planck’s hypothesis is used to explain quite a few phenomena not otherwise following a classical explanation. QUANTUM OF LIGHT Light carries a specific (discrete) amount of energy: 𝐸 = ℎ𝑓 Planck’s hypothesis connects the wave nature of light with the particle nature of photons (i.e. wave-particle duality) Helps explain the photoelectric effect, which cannot be explained classically. #1 Calculate the energy of a photon of ultraviolet light, wavelength 3.00 x 10-7 m. Express your answer in both Joules and in electronvolts. 𝒉𝒄 𝑬 = 𝒉𝒇 = 𝝀 (𝟔. 𝟔𝟑 × 𝟏𝟎−𝟑𝟒 )(𝟑. 𝟎𝟎 × 𝟏𝟎𝟖 ) 𝑬= (𝟑. 𝟎 × 𝟏𝟎−𝟕 ) 𝑬 = 𝟔. 𝟔𝟑 × 𝟏𝟎−𝟏𝟗 𝑱 𝟔. 𝟔𝟑 × 𝟏𝟎−𝟏𝟗 𝑱 𝟏 𝒆𝑽 × = 𝟒. 𝟏𝟒 𝒆𝑽 −𝟏𝟗 𝟏. 𝟔𝟎 × 𝟏𝟎 𝑱 SIMULATION LAB Extra Review: Watch this video after finishing your lab and after reviewing your notes: https://www.youtube.com/watch?v=ubkNGwu_ 66s PHOTOELECTRIC EFFECT Work Function (φ) the minimum amount of energy that must be absorbed by an electron in order to be emitted from a metal Depends on the metal! Threshold frequency (f0)the lowest frequency that will allow electrons to be emitted from a metal’s surface. ℎ𝑓 = 𝐸𝑘 + 𝜑 E = kinetic energy of emitted electron At the threshold frequency, the kinetic energy of the emitted electron = 0 eV STOPPING VOLTAGE (STOPPING POTENTIAL) The amount of potential needed to just stop the flow of electrons through the vacuum tube. The resulting current becomes 0 A The stopping voltage is equivalent to the amount of kinetic energy of the emitted electrons 𝑉𝑠 = 𝐸𝑘 GRAPHING PHOTOELECTRIC EFFECT