The Acuity Trial - Clinical Trial Results
advertisement

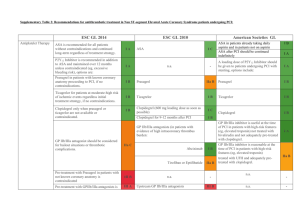
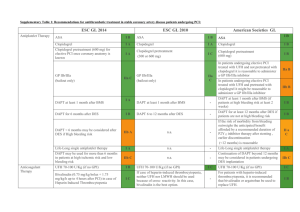
OPTIMAL UPSTREAM ANTITHROMBIN THERAPY IN NSTE ACS PATIENTS MANAGED IN THE CARDIAC CATH LAB: DOES IT MATTER WHICH AGENT IS STARTED IN THE ED? Charles V. Pollack, Jr., M.A., M.D., FACEP, FAAEM Department of Emergency Medicine Pennsylvania Hospital, Philadelphia Steven V. Manoukian, Gregg W. Stone, Judd E. Hollander, Chadwick Miller, Deborah B. Diercks, W. Frank Peacock, Gerard X. Brogan, Charles L. Emerman, Andra Blomkalns, W. Brian Gibler, Ivan Rokos, David Larson, and James W. Hoekstra NSTE ACS: Optimal Therapy, 2006 Braunwald E, Antman EM, Beasley JW, et al: ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina). J Am Coll Cardiol 2000;36:970-1062 (2002 update at www.acc.org; summary in Circulation 2002;106:1893-1900) Pollack CV, Roe MT, Peterson ED: 2002 Update to the ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: Implications for emergency department practice. Ann Emerg Med 2003;41:355-69. Hospital Care: Anti-Thrombotic Therapy I IIa IIb III Immediate aspirin Clopidogrel, if aspirin contraindicated Heparin (IV unfractionated, LMW) with antiplatelet agents listed above Enoxaparin preferred over UFH unless CABG is planned within 24 hours Braunwald et al, Circulation 2002;106:1893-1900 Acute Medication Use in High-Risk NSTE Patients: CRUSADE Within first 24 hours in patients without contraindications 100% 96% 90% 82% % Use 80% 54% 60% 47% 40% 20% 0% Aspirin Beta Blockers Heparin (LMW+UFH) CRUSADE DATA: Quarter 4, 2004 – Quarter 3, 2005 (n=35,897) GP llb-llla Inhibitors Clopidogrel Hospital Care Conservative vs. Invasive Strategies I IIa IIb III Early invasive strategy in high-risk patients with any of the following: - Recurrent ischemia, despite meds - Elevated Troponin I or T - New ST-segment depression - New CHF symptoms - High-risk stress test findings - LV dysfunction (EF < 40%) - Hemodynamic instability, sustained VT - PCI within 6 months, prior CABG Braunwald et al, Circulation 2002;106:1893-1900 Hospital Care Conservative vs. Invasive Strategies I IIa IIb III Either strategy in low- to moderate-risk patients without contraindications to revascularization Early invasive strategy for patients with repeated ACS presentations, without high-risk features or ongoing ischemia Braunwald et al, Circulation 2002;106:1893-1900 Invasive Cardiac Procedures: CRUSADE (among patients without contraindications to cath) 100% Median Times 82% 80% % Use 65% 60% 51% • Cath - 22 hrs • PCI - 21 hrs • CABG - 69 hrs 37% 40% 20% 12% 0% Cath Cath < 48 hr CRUSADE data, unpublished, March 2006 PCI PCI < 48 hr CABG ACUITY Study (ACC, March 2006) UFH or Enoxaparin + IIb/IIIa Moderate and high risk ACS Aspirin in all Clopidogrel dosing and timing per local practice R* Bivalirudin + IIb/IIIa Bivalirudin Alone Angiography within 72h Moderate and high risk unstable angina or NSTEMI undergoing an invasive strategy (N = 13,819) *Stratified by pre-angiography thienopyridine use or administration ACUITY Design. Stone GW et al. AHJ 2004;148:764–75 Medical management PCI CABG Primary Endpoint Measures (ITT) UFH/Enoxaparin + GPI vs. Bivalirudin Alone 30 day events (%) UFH/Enoxaparin+GPI (N=4603) PNI <0.0001 PSup = 0.015 11.7% Bivalirudin alone (N=4612) PNI = 0.011 PSup = 0.32 PNI <0.0001 PSup <0.0001 10.1% 7.3% 7.8% 5.7% 3.0% Net clinical outcome Ischemic composite Major bleeding ACUITY More than 99% of patients in ACUITY were taken to the cath lab, at a median time of 19.6 hours after arrival. One-third of these patients were randomized to receive bivalirudin monotherapy. The median time from randomization to angiography/intervention was approximately 5 hours. Because of their assessed ischemic risk, 64.1% of patients enrolled in ACUITY were treated with either UFH or enoxaparin prior to randomization. Consistently, 63.9% of patients randomized to bivalirudin monotherapy were pretreated . . . 40.5 % with UFH 25.4 % with enoxaparin Rationale and Hypothesis In contemporary practice, bivalirudin is not ordinarily used outside the cardiac cath lab. Patients with NSTE ACS and high risk features are typically administered an anticoagulant in the ED as empiric therapy. Whether UFH or enox is used in this setting is an issue of local preference and policy. Hypothesis: The nature of upstream anticoagulant therapy—UFH or enox—will not affect outcomes of NSTE ACS patients who subsequently receive bivalirudin in the cath lab. ACUITY: Methodology Patients were excluded from the study if they had received more than one dose of enox prior to potential randomization. Patients who had received one dose of enox were eligible for inclusion. Randomized therapy initiated 12h after enox dose Patients who had received UFH were eligible for inclusion. 30-minute wash-out period ACUITY: Results Of 13,819 patients enrolled, 4,612* were randomized to receive bivalirudin monotherapy. Of these: 1,658 received no prior anticoagulant 1,773 received UFH prior to randomization 1,073 received enox prior to randomization 97 received both UFH and enox and were excluded from this analysis. * 11 pts did not have complete data available Patient Characteristics BIV—no AT (N=1,658) UFH→BIV (N=1,773) Enox→BIV (N=1,073) Arrival to cath [median, hrs] 18.82 16.85 23.67* % to PCI 54.8% 59.7% 55.2% % to CABG 9.8% 10.7% 12.0% % to MM 35.5% 29.6% 32.8% % pre-rand GPI 2.4% 9.6%* 6.8%* * P value < 0.001 Ischemic Outcomes BIV—no AT (N=1,658) UFH→BIV (N=1,773) Enox→BIV (N=1,073) 30d death 1.4% 1.4% 2.4% 30d MI 4.8% 5.8% 5.5% Unplanned revasc 2.1% 2.7% 2.3% Composite 6.8% 8.3% 8.7% All comparisons NS - before and after adjustment for baseline characteristics Bleeding Outcomes BIV—no AT (N=1,658) UFH→BIV (N=1,773) Enox→BIV (N=1,073) TIMI Major non-CABG Bleed (%) 0.9% 1.0% 1.0% Protocol Non-CABG Major Bleed (%) 3.2% 2.9% 3.1% Any TIMI non-CABG Bleed (%) 3.7% 4.2% 4.3% Protocol Non-CABG Minor Bleed (%) 13.3% 11.3% 14.2% Non-CABG Transfusions (%) 1.6% 1.7% 1.4% All comparisons NS - before and after adjustment for baseline characteristics Net Clinical Outcome BIV - no AT vs. UFH →BIV vs. Enox →BIV 30 day events (%) BIV - No AT (N=1658) UFH to BIV (N=1773) 10.4% 9.1% Net Clinical Outcome All comparisons NS Enox to BIV (N=1073) 11.4% Primary Outcomes: Summary by PreRandomization AT BIV - no AT vs. UFH →BIV vs. Enox →BIV 30 day events (%) BIV - No AT (N=1658) UFH to BIV (N=1773) Enox to BIV (N=1073) 11.4% 10.4% 9.1% 8.3% 8.7% 6.8% 3.2% 2.9% 3.1% Net Clinical Outcome All comparisons NS Composite Ischemia Major Bleeding Outcomes in High-Risk Patients Elevated Biomarker and/or ST-segment changes BIV—no AT UFH→BIV Enox→BIV N=989 (59.7%) N=1,279 (72.1%) N=827 (77.1%) Composite ischemia 8.3% 9.1% 9.8% Protocol Non-CABG Major Bleed 3.6% 3.6% 3.7% Protocol Non-CABG Minor Bleed 13.8% 12.3% 14.6% Non-CABG Transfusions 1.6% 2.2% 1.8% Net clinical outcome 10.9% 11.9% 13.1% All comparisons NS Primary Outcomes – High Risk Patients BIV - no AT vs. UFH →BIV vs. Enox →BIV 30 day events (%) BIV - No AT (N=989) UFH to BIV (N=1279) 13.1% 11.9% 10.9% 8.3% 9.1% Enox to BIV (N=827) 9.8% 3.6% 3.6% 3.7% Net Clinical Outcome All comparisons NS Composite Ischemia Major Bleeding Caveats Further analysis of the ACUITY data will ascertain the impact of duration of therapy with bivalirudin on outcomes, as well as the relationship between upstream GPI use and risk as perceived in the ED. There will also be a comprehensive economic analysis. The dose of bivalirudin used in ACUITY for precath management is not a labeled dose. The washout periods for prior AT therapy mandated in the study may not be scrupulously followed in contemporary practice. Conclusions The efficacy and safety of bivalirudin in patients with moderate and high risk NSTE ACS from the ACUITY trial is not significantly influenced by prior AT with UFH or enox. Prior UFH or enox does not significantly compromise the net clinical outcomes achieved in patients subsequently converted to bivalirudin for interventional management. Study Medications Anti-thrombin agents (started pre angiography) UF Heparin U/Kg Enoxaparin mg/Kg Bivalirudin mg/kg Bolus 60 1.0 sc bid 0.1 iv Infusion/h 121 PCI CABG Medical mgt 1 0.25 iv ACT 200-250s 0.30 iv bolus2 0.75 iv bolus3 0.50 bolus iv 1.75/h infusion iv4 Per institution Per institution Per institution5 None6 None6 None6 Target aPTT 50-75 seconds If last enoxaparin dose ≥8h - <16h before PCI; 3 If maintenance dose discontinued or ≥16h from last dose 4 Discontinued at end of PCI with option to continue at 0.25mg/kg for 4-12h if GPIIb/IIIa inhibitor not used 5 Bivalirudin option for off-pump same as PCI dose. For on-pump bivalirudin discontinued 2 hours before 6 Option to continue with pre-PCI anti-thrombotic regimen at physician discretion 2