The Nucleus
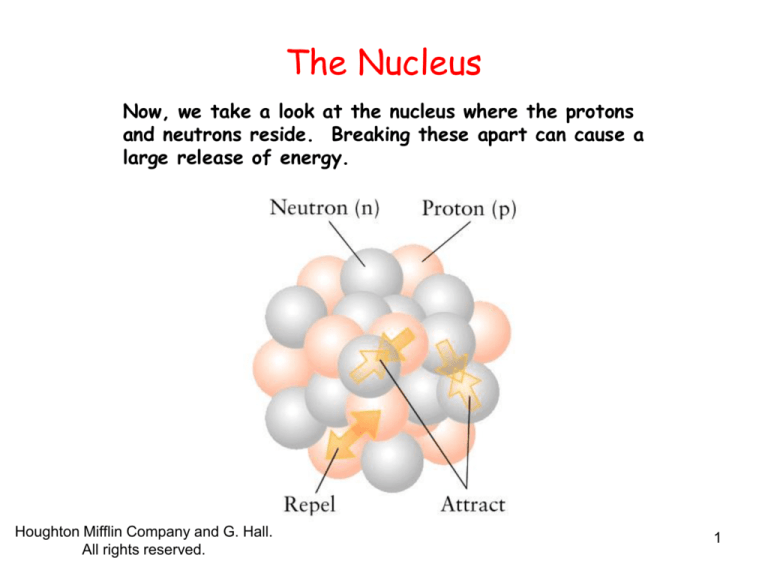
Now, we take a look at the nucleus where the protons
and neutrons reside. Breaking these apart can cause a
large release of energy.
Houghton Mifflin Company and G. Hall.
All rights reserved.
1
Table 24.1
Houghton Mifflin Company and G. Hall.
All rights reserved.
2
Fig. 24.1
Three types of
particles.
Houghton Mifflin Company and G. Hall.
All rights reserved.
3
Shielding
Houghton Mifflin Company and G. Hall.
All rights reserved.
4
Nuclear Nomenclature
Nuclides need to know the symbols for protons and
neutrons and how they add up to form isotopes
(same atomic number, different atomic mass) H
(hydrogen), D (deuterium), T (tritium).
Nuclide designation:
A
Z
E
234
90
Th
where E is the element, A is the mass number =
number of protons + neutrons (isotope), and Z is
the atomic number (number of protons).
Also, we sometimes write the isotope as Fe-55,
Co-60, Pb-206, etc. In the nucleus, the protons
and neutrons are called nucleons.
Houghton Mifflin Company and G. Hall.
All rights reserved.
5
A = sum of neutrons and protons i.e. isotope.
Z = number of protons.
N = number of neutrons.
Houghton Mifflin Company and G. Hall.
All rights reserved.
6
A
Z
A = sum of neutrons and protons i.e. isotope.
Z = number of protons.
N = number of neutrons.
Houghton Mifflin Company and G. Hall.
All rights reserved.
7
N
Alpha (a ) particle emission
238
92
U 234
90Th
4
2
He
notice both sides equal
with mass and atomic number.
Houghton Mifflin Company and G. Hall.
All rights reserved.
8
Beta (b) particle emission
1
0
n p e
1
1
Th
234
90
Houghton Mifflin Company and G. Hall.
All rights reserved.
0
-1
234
91
Pa e
0
-1
9
Gamma ray (g) emission, atom is radioactive
Th
230m
90
Th g
230
90
m is for metastable state.
g ray is electromagnetic radiation i.e. photon.
Houghton Mifflin Company and G. Hall.
All rights reserved.
10
Positron (+e) emission
1
1
p n e
1
0
26
13
0
1
Al Mg e
26
12
0
1
(electron with positive
charge i.e. anti matter)
Houghton Mifflin Company and G. Hall.
All rights reserved.
11
Electron capture
1
1
p e n
125
53
0
-1
1
0
I e Te
0
-1
125
52
In the nucleus, a proton
and electron react.
Houghton Mifflin Company and G. Hall.
All rights reserved.
12
Fig. 24.2
Houghton Mifflin Company and G. Hall.
All rights reserved.
13
Prob. 24.1
Houghton Mifflin Company and G. Hall.
All rights reserved.
14
Radioactive Decay
Houghton Mifflin Company and G. Hall.
All rights reserved.
15
Radioisotope dating
We can use the first order rate law
and decay of certain isotopes to
determine how old certain objects are.
This method is used extensive for
radiocarbon dating which is based on
the decay of 14C.
Houghton Mifflin Company and G. Hall.
All rights reserved.
16
Rate of Decay
Rate (A) = kN
The rate of decay is proportional to the
number of nuclides. This represents a
first-order process.
The SI unit of radioactivity is the
becquerel (Bq). A larger unit is the curie
(Ci) = 3.7E10 d/s.
Houghton Mifflin Company and G. Hall.
All rights reserved.
17
Half-Life
. . .
the time required for the
number of nuclides to reach half
the original value (N0/2).
t1/ 2
Houghton Mifflin Company and G. Hall.
All rights reserved.
ln(2) 0.693
k
k
18
Radiodating
This first order decay is very helpful in
carbon or other datings. ln (At/Ao) = -lt.
You can see that if we know the N at
various times, we can determine t the age
of an object provided that the half-life is
not to long or to short.
In the upper atmosphere, the reaction
takes place due to cosmic radiation that
results in neutrons. The nuclear reaction:
14
7
Houghton Mifflin Company and G. Hall.
All rights reserved.
N n C H
1
0
14
6
1
1
19
Houghton Mifflin Company and G. Hall.
All rights reserved.
20
Carbon-14 Dating
Houghton Mifflin Company and G. Hall.
All rights reserved.
21
Brigham Young
researcher Scott
Woodward taking
a bone sample
for carbon-14
dating at an
archeological site
in Egypt.
Fig. 24.4
Houghton Mifflin Company and G. Hall.
All rights reserved.
23
Table 24.5
Houghton Mifflin Company and G. Hall.
All rights reserved.
24
Radiation Units
Houghton Mifflin Company and G. Hall.
All rights reserved.
25
Table 24.7
Houghton Mifflin Company and G. Hall.
All rights reserved.
26
Houghton Mifflin Company and G. Hall.
All rights reserved.
27
Fig. 24.13
Houghton Mifflin Company and G. Hall.
All rights reserved.
28
Energy and Mass related to radioactive
decay of uranium
When a system gains or loses energy it also
gains or loses a quantity of mass.
E = mc2
m = mass defect E m
2
c
E = change in energy
If E = (exothermic), mass is lost from the
system.
Houghton Mifflin Company and G. Hall.
All rights reserved.
29
Fig. 24.16
Houghton Mifflin Company and G. Hall.
All rights reserved.
30
Nuclear waste storage (2 Engineers)
Houghton Mifflin Company and G. Hall.
All rights reserved.
31
Fig. B24.1
Houghton Mifflin Company and G. Hall.
All rights reserved.
32
Lecture summary
Know the different types of radiation.
Decay of different radiation.
Rate law and its use in radiodating.
Radioisotopes are used extensively in
medicine and in industrial applications.
Radiation is safe provided the proper
safety measures are taken.
Houghton Mifflin Company and G. Hall.
All rights reserved.
33
Notes
Please check the class website
www.rutchem.rutgers.edu
For further developments such as
practice exam and exam notes.
Have a safe and pleasant summer.
Houghton Mifflin Company and G. Hall.
All rights reserved.
34


