Standard Application Form
advertisement
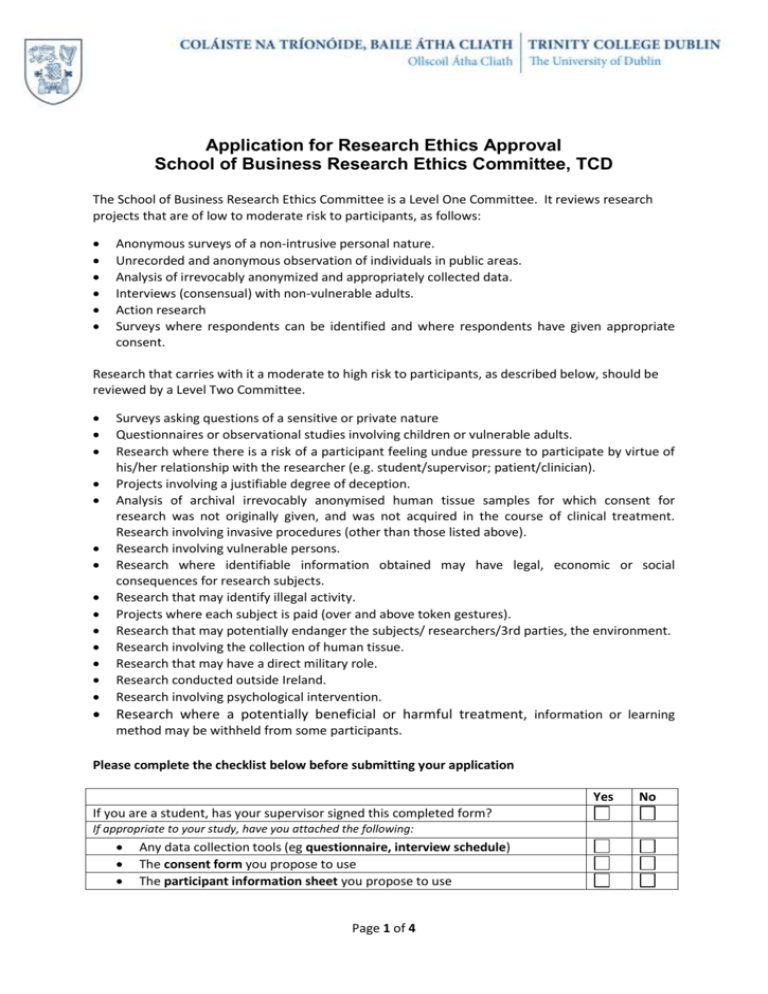
Application for Research Ethics Approval School of Business Research Ethics Committee, TCD The School of Business Research Ethics Committee is a Level One Committee. It reviews research projects that are of low to moderate risk to participants, as follows: Anonymous surveys of a non-intrusive personal nature. Unrecorded and anonymous observation of individuals in public areas. Analysis of irrevocably anonymized and appropriately collected data. Interviews (consensual) with non-vulnerable adults. Action research Surveys where respondents can be identified and where respondents have given appropriate consent. Research that carries with it a moderate to high risk to participants, as described below, should be reviewed by a Level Two Committee. Surveys asking questions of a sensitive or private nature Questionnaires or observational studies involving children or vulnerable adults. Research where there is a risk of a participant feeling undue pressure to participate by virtue of his/her relationship with the researcher (e.g. student/supervisor; patient/clinician). Projects involving a justifiable degree of deception. Analysis of archival irrevocably anonymised human tissue samples for which consent for research was not originally given, and was not acquired in the course of clinical treatment. Research involving invasive procedures (other than those listed above). Research involving vulnerable persons. Research where identifiable information obtained may have legal, economic or social consequences for research subjects. Research that may identify illegal activity. Projects where each subject is paid (over and above token gestures). Research that may potentially endanger the subjects/ researchers/3rd parties, the environment. Research involving the collection of human tissue. Research that may have a direct military role. Research conducted outside Ireland. Research involving psychological intervention. Research where a potentially beneficial or harmful treatment, information or learning method may be withheld from some participants. Please complete the checklist below before submitting your application Yes If you are a student, has your supervisor signed this completed form? If appropriate to your study, have you attached the following: Any data collection tools (eg questionnaire, interview schedule) The consent form you propose to use The participant information sheet you propose to use Page 1 of 4 No Name of Applicant Academic Supervisor/ Lead Researcher For students this is the name of your supervisor. For staff this is the PI if different from the applicant Discipline Title of project Timeframe of research Provide a brief timetable of the proposed research, particularly indicating data collection. Funder Where research is funded, give details of the funder and indicate whether the funder requires that ethical approval is secured for this project Requirement for Ethical Approval a) Where another party has explicitly required ethical approval for this project, please provide details. b) If you are required to seek further ethical approval from another committee after this application, please provide details. Purpose of research Provide a summary of the research, written in terms that a non-specialist would understand. Justification for the research Page 2 of 4 Indicate the contribution that the research is anticipated to make. Participants in the research Provide details of the population to be studied, and sampling procedures to be used. Recruitment procedures Include an explanation of any incentives and/or compensation (financial or otherwise) to be offered to participants. Informed consent Outline the information that will be provided to potential participants, and procedures for gaining consent (if this will be in printed form, please supply a copy of it). Methods Outline the methods that will be used for data collection and analysis and provide interview or survey questions where these are being used. Confidentiality, anonymity, and data storage Provide an explanation of any measures that will be put in place to preserve confidentiality and anonymity, including an explicit explanation of secure data storage and disposal plans. Note that there may be a need to store data for 5 years (and sometimes more) after completion of the project. Ethical considerations and potential risks to participants Where potential risks to participants Page 3 of 4 may be present, explain any steps that will be taken to minimize these and any additional support that might be used should the need arise. Published ethical guidelines to be followed Identify professional code(s) of practice and/or ethical guidelines relevant to the research. Travel outside Ireland Provide information on any travel to be undertaken outside Ireland. Any travel planned for countries with heightened risk should be given detailed explanation and justification. Self-care Identify any significant risk issues to yourself as researcher and any selfcare planning that you will use to address these. Signature of applicant: I declare that I have read the TCD Ethics Policy and will follow the guidelines therein. For student applications: I also confirm that this application has already been reviewed and is supported by my supervisor. Signature of the Chair of the School of Business Research Ethics Committee1 In my capacity as Chair of the School’s Ethics Committee, I confirm that this project has been approved by the School’s Ethics Committee 1 Signature: [A typed name is acceptable as long as it is submitted from applicant’s TCD email address, as verified by Chair of Ethics Committee below] Date: Name of Supervisor: [For student applications] Signature: Date: This application form was adapted from that used by the School of Social Sciences & Philosophy, TCD. Page 4 of 4








