MOON Shoulder Fellows Talk
advertisement

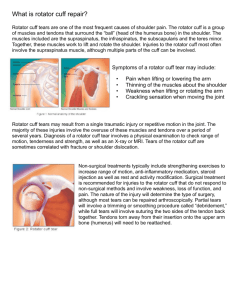
John E. Kuhn, MD Tara T. Holmes, PA-C, MPH What is ? MOON - Multicenter Orthopaedic Outcome Network. MOON Knee, est. in 2001, is a consortium of 7 sites and 17 surgeons studying outcomes following anterior cruciate ligament reconstruction. MOON Shoulder, est. in 2004 and modeled after MOON Knee, is a consortium of 9 sites and 17 surgeons studying disorders of the shoulder Vanderbilt Sports Medicine functions as the Coordinating Center for MOON. Outcomes Research MOON Shoulder Ultimate Goal: Study Features that Influence Success of Rotator Cuff Repair MOON Shoulder Consortium: 9 Sites - 17 Surgeons Vanderbilt Orthopaedic Institute Warren R. Dunn, MD, MPH John E. Kuhn, MD Kurt P. Spindler, MD James Carey, MD Washington University – St. Louis Rick W. Wright, MD Robert Brophy, MD University of Colorado Eric C. McCarty, MD Armando F. Vidal, MD University of Iowa Brian R. Wolf, MD, MS Hospital for Special Surgery Robert G. Marx, MD, MSc, FRCSC Ohio State University Grant L. Jones, MD Julie Y. Bishop, MD David C. Flanigan, MD University of California at San Francisco C. Benjamin Ma, MD Orthopedic Institute Keith M. Baumgarten, MD Knoxville Orthopedic Clinic Edwin E. Spencer Jr, MD Brian Holloway, MD Participating Sites: Support Staff Orthopedic Institute Kari Caspers Dayna Semchenko, PA-C Patrick Heiser, PA-C University of Iowa Carla Britton Andrea Wilson, PA-C Ohio State University Angela Pedroza Melissa Bowlby Justin Long Wendy McGeehan This research would not be possible without our tremendous support staff! Hospital for Special Surgery Jessica Ryu University of California at San Francisco Emily Keifa Knoxville Orthopedic Clinic Lori Sharp, PA-C Mary Cate Andrea Parton University of Colorado Paula Langner Washington University – St. Louis Linda Burnworth Michele Cooper Vanderbilt Sports Medicine (Coordinating Center) Biostatistics Frank Harrell, Ph.D Warren R. Dunn, MD, MPH Zhouwen Liu, MS C. Thomas Dupont, BS Research Coordinator Tara T. Holmes, PA-C, MPH Research Analyst Brooke E. Rode Additional Research Staff Angela Combs, RN Angela Keen Sarah Editorial Assistance Lynn Sykes Cain Features to Predict Success with Nonoperative Treatment of Patients with Fullthickness Rotator Cuff Tears (Kuhn) NIH 5K23AR052392-02 (Dunn) Clinical Scholars in Epidemiology (Dunn) Ongoing Development 1. Monthly Conference calls to review/discuss issues related to current study and to discuss additional/future studies 2. Group meets in-person three times per year 3. Manual of Operations gets reviewed and updated as necessary Phase I-Reducing Variables • Common Methods of Describing Pathology/Findings/Independent Variables – Validated Data Collection Forms – Agreement Studies • Common Practice Patterns – Criteria to Order MRI – Surgical Indications – Postoperative Care Phase II – Data Collection • • • • • Prospective Cohort Study 2000 patients Long F/U with MRI NIH Funding Identify Controllable Features that Influence Failure of Rotator Cuff Repair Phase III – Randomized Trials The MOON Shoulder consortium met 7 times (5/04 – 10/06) with the following goals 1. Formulate research questions of interest for the study of rotator cuff disease 2. Develop standardized criteria for ordering an MRI 3. Develop a consensus on the standard x-ray and MRI imaging techniques 4. Select and develop outcomes instruments Consensus No Data in Literature The MOON Shoulder consortium met 7 times (5/04 – 10/06) with the following goals 1. 2. 3. Develop standardized criteria for ordering an MRI Develop a consensus on the standard x-ray and MRI imaging techniques Select and develop outcomes instruments Development of Data Collection Forms and Database Patient Form Physical Exam Form Surgical Data Collection Form Systematic Reviews • Nonoperative Physical Therapy Program • Surgical Indications in Treatment of Rotator Cuff Disease • Postoperative Care – Cryotherapy – Physical Therapy – CPM Original Research • Agreement Study on Describing MRI Findings • Agreement Study on Describing Intraarticular Pathology • Study on Nonoperative Treatment for Common Surgical Indications Interobserver agreement studies 1. Interobserver agreement in the classification of rotator cuff tears (Kuhn et al AJSM 2007) – LOE: II • • • • 12 fellowship trained orthopaedic surgeons reviewed arthroscopy videos from 30 patients and classified them using 6 different classification systems Interobserver agreement determined by kappa analysis High when distinguishing full-thickness from partial-thickness tears (0.95, K=0.85) High when identifying the side of involvement of partial thickness tears (0.95, K=0.85) Classification of Video Cuff Disease • Inter-observer agreement was high when distinguishing between full thickness and partial thickness tears (0.95, κ=0.85). • The investigators agreed on the side (articular vs. bursal) of involvement for partial thickness tears (observed agreement 0.92, κ=0.85), but could not agree when classifying the depth of the partial thickness tear (observed agreement 0.49, κ=0.19). • The best agreement for full thickness tears was seen when tear was classified by topography in the frontal plane (observed agreement 0.70, κ=0.54). Interobserver agreement studies 2. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging (Spencer et al 2007 – accepted AJSM June 2007) – LOE: II • • 10 fellowship trained orthopaedic surgeons reviewed 27 MRIs with varying degrees of rotator cuff pathology Interobserver agreement determined by kappa analysis Interobserver agreement studies 2. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging • Assessed MRIs for: • the ability to detect FT vs. PT tears, • acromion morphology, • AC joint spurs or signal changes, • individual muscle fatty infiltration and atrophy, • biceps pathology, • size and grade partial thickness tears, • acromiohumeral distance, • number of tendons involved, • amount of retraction for full-thickness tears, and • size of full thickness tears Interobserver agreement studies 2. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging • • Highest when distinguishing full-thickness from partial-thickness tears (0.95, K=0.77) Moderate when identifying the number of tendons involved (0.95, K=0.55) Variable Teres minor quantity Observed Agreement (percentage) Kapp a 0.9 0 Full thickness vs. Partial Thickness 0.89 0.77 AC joint signal change (increased) 0.78 0.33 Subscapularis quantity 0.78 0.04 Number of tendons involved 0.72 0.55 Side of partial thickness tear 0.72 0.44 Infraspinatus quantity 0.72 0.22 Biceps tear 0.68 0.19 AC joint spur 0.66 0.32 Degree of retraction 0.63 0.44 0.6 0.2 Supraspinatus quantity 0.59 0.25 Acromiohumeral distance 0.52 0.26 0.5 0.16 Partial thickness tear grade 0.46 -0.11 Acromial morphology(coronal) 0.43 0.06 Size of tear (sagital) 0.42 0.26 Size of tear (coronal) 0.42 0.24 Muscle quality(Goutallier) 0.36 0.1 Biceps signal change(increased) Acromial morphology(sagital) Additional Manuscripts • Indications for Repair of Full-thickness Rotator Cuff Tears (Review) Wolf BR, Dunn WR, Wright R. AJSM June 2007; 35(6): 1007-16. 2. Rotator Cuff Repair Rehabilitation: A Level I and Level II Systematic Review Baumgarten KM, Vidal AF, Wright RW. Submitted to AJSM July 2007 3. Features of Rehabilitation for Rotator Cuff Disease, A Systematic Review Kuhn JE in preparation Prospective Cohort Study Features to predict success with nonoperative treatment for full thickness rotator cuff tears. Specific Aims 1. Study the effect of historical information on predicting success (determined by pain relief & patient satisfaction) of nonoperative treatment using an EBM based PT program 2. Study the effect of physical exam findings on predicting success of nonoperative treatment using an EBM based PT program 3. Study the effect of the severity of the rotator cuff pathology (using standardized MRI protocols) on predicting success of nonoperative treatment using an EBM based PT program. Prospective Cohort Study Inclusion Criteria: • All patients (18-100 yrs. of age) • MRI documented full-thickness (FT) rotator cuff tear Exclusion Criteria • • • • • • Acute rotator cuff tear (RCT) Associated dislocation Associated fracture Systemic Rheumatologic disease Dementia or inability to complete questionnaires Bilateral Cuff tears Prospective Cohort Study Enrollment (began Jan. 2007) • We are asking sites to keep track of ALL full-thickness rotator cuff tears on a standard enrollment log • Total number of FT cuff tears • Total number who DO NOT fit I/E criteria • Total number who fit I/E criteria and consent • Total number who fit I/E criteria and decline to consent Prospective Cohort Study Patients presenting WITHOUT an MRI are evaluated in a standard manner to determine if an MRI is indicated to rule out a rotator cuff tear. Patients with an MRI documented FT cuff tear are approached about participation by the attending physician or a member of the research staff. Outcome measures - a self-administered questionnaire, a rehab diary and a modified ASES exam form. Outcome Measure Self-administered questionnaire* Rehab Diary † Physician Exam form (modified ASES) Time Point 12 wk 2 year 5 year 10 year Initial 6 wk X X X X X X X X X X X * A compilation of the SF-12v2, the ASES, the WORC, the Marx Activity Scale, the SANE, patient expectations and the Self-Administered Comorbidity Questionnaire. † Consisted of two questions: Did you go to formal physical therapy? Did you do a home exercise program? Study to Date • 1/3 of patients required enrolled • 15% drop out rate • Early data suggests good response to PT Immediate Future of MOON Shoulder • Continue to Collect Data on Nonoperative Cohort Patients (N=380) • Begin NIH Grant for Surgical Treatment Cohort • Continue to Explore Agreement Studies and Develop Systematic Reviews on Other Shoulder Topic A Few Years from Now • Randomized Controlled Trials on Interventions to Improve Success with Rotator Cuff Repair • Cohort Studies of Other Shoulder Disorders – Instability Summary • EBM is the Future of Orthopaedics and Sports Medicine • Level-4 Case Series are not worth your time unless the condition is very rare • Multicenter Cohort Studies or Trials is the best way to contribute in a clinically meaningful way! Thanks!