Electronic Supplementary Material: S.D. Ling et al. Global regime
advertisement
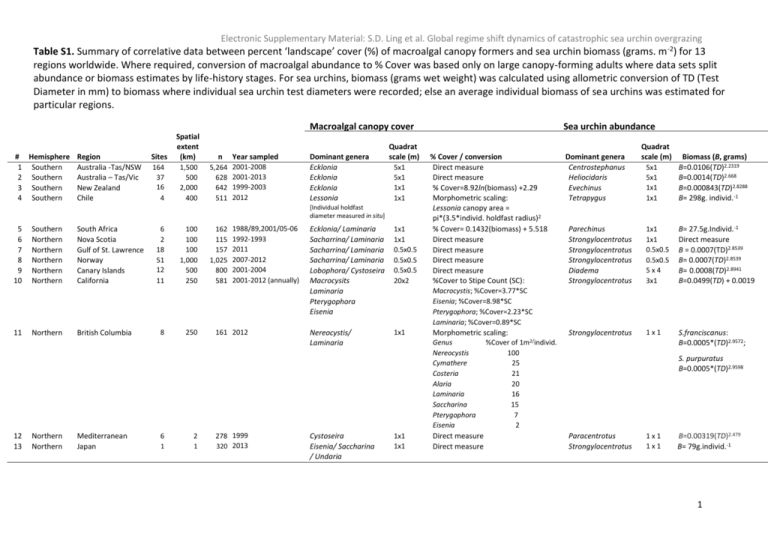
Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing Table S1. Summary of correlative data between percent ‘landscape’ cover (%) of macroalgal canopy formers and sea urchin biomass (grams. m-2) for 13 regions worldwide. Where required, conversion of macroalgal abundance to % Cover was based only on large canopy-forming adults where data sets split abundance or biomass estimates by life-history stages. For sea urchins, biomass (grams wet weight) was calculated using allometric conversion of TD (Test Diameter in mm) to biomass where individual sea urchin test diameters were recorded; else an average individual biomass of sea urchins was estimated for particular regions. Macroalgal canopy cover # Hemisphere Region 1 Southern Australia -Tas/NSW 2 Southern Australia – Tas/Vic 3 Southern New Zealand 4 Southern Chile Sites Spatial extent (km) n 164 37 16 5 4 1,500 500 2,000 400 5,264 628 642 511 Year sampled 2001-2008 2001-2013 1999-2003 2012 Dominant genera Ecklonia Ecklonia Ecklonia Lessonia Quadrat scale (m) 5x1 5x1 1x1 1x1 [Individual holdfast diameter measured in situ] 5 6 7 8 9 10 11 12 13 Southern Northern Northern Northern Northern Northern Northern Northern Northern South Africa Nova Scotia Gulf of St. Lawrence Norway Canary Islands California British Columbia Mediterranean Japan 6 2 18 51 12 5 11 8 100 100 100 1,000 500 250 250 6 2 1 1 162 115 157 1,025 800 581 1988/89,2001/05-06 1992-1993 2011 2007-2012 2001-2004 2001-2012 (annually) 161 2012 278 1999 320 2013 Ecklonia/ Laminaria Sacharrina/ Laminaria Sacharrina/ Laminaria Sacharrina/ Laminaria Lobophora/ Cystoseira Macrocysits Laminaria Pterygophora Eisenia 1x1 1x1 0.5x0.5 0.5x0.5 0.5x0.5 20x2 Nereocystis/ Laminaria 1x1 Cystoseira Eisenia/ Saccharina / Undaria Sea urchin abundance % Cover / conversion Direct measure Direct measure % Cover=8.92ln(biomass) +2.29 Morphometric scaling: Lessonia canopy area = pi*(3.5*individ. holdfast radius)2 % Cover= 0.1432(biomass) + 5.518 Direct measure Direct measure Direct measure Direct measure %Cover to Stipe Count (SC): Dominant genera Centrostephanus Heliocidaris Evechinus Tetrapygus Quadrat scale (m) 5x1 5x1 1x1 1x1 Biomass (B, grams) B=0.0106(TD)2.2319 B=0.0014(TD)2.668 B=0.000843(TD)2.8288 B= 298g. individ.-1 Parechinus Strongylocentrotus Strongylocentrotus Strongylocentrotus Diadema Strongylocentrotus 1x1 1x1 0.5x0.5 0.5x0.5 5x4 3x1 B= 27.5g.Individ.-1 Direct measure B = 0.0007(TD)2.8539 B= 0.0007(TD)2.8539 B= 0.0008(TD)2.8941 B=0.0499(TD) + 0.0019 Strongylocentrotus 1x1 S.franciscanus: B=0.0005*(TD)2.9572; Macrocystis; %Cover=3.77*SC Eisenia; %Cover=8.98*SC Pterygophora; %Cover=2.23*SC Laminaria; %Cover=0.89*SC Morphometric scaling: Genus Nereocystis Cymathere Costeria Alaria Laminaria Saccharina Pterygophora Eisenia 1x1 1x1 Direct measure Direct measure %Cover of 1m2/individ. 100 25 21 20 16 15 7 2 S. purpuratus B=0.0005*(TD)2.9598 Paracentrotus Strongylocentrotus 1x1 1x1 B=0.00319(TD)2.479 B= 79g.individ.-1 1 Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing Table S2. Summary of observational and manipulative experiments detailing the magnitude and directional response of macroalgal habitat (both forward and reverse regime shifts) following change in sea urchin abundance. Studies were included if stated magnitudes in the macroalgal response and sea urchin abundance could be standardized as percentage cover (%) for macroalgae and biomass (grams. m-2) for sea urchins respectively. Experiments involved sudden changes in urchin biomass density between “Start” & “End”: experimenters either reduced or increased urchins to a target density; or in the case of observational studies, either disease (reverse shifts) or local aggregation (forward shifts) also caused sudden change in urchin density. But see exception (study #43) in footnote below. # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 Hemisphere Northern Southern Southern Northern Southern Southern Northern Southern Southern Northern Northern Northern Southern Southern Northern Northern Northern Northern Northern Southern Southern Southern Southern Southern Southern Southern Southern Southern Southern Southern Southern Northern Northern Southern Northern Northern Southern Northern Northern Northern Northern Northern Southern Northern Southern Northern Northern Northern Southern Northern Northern Northern Northern Northern Northern Northern Northern Region New England Australia Australia British Columbia Australia Australia California Australia Australia Nova Scotia Nova Scotia Nova Scotia Australia Australia Nova Scotia Canary Islands Canary Islands Nova Scotia Alaska Australia New Zealand Australia Australia Australia Australia Australia Australia Australia Australia New Zealand New Zealand British Columbia British Columbia Australia New England Nova Scotia Australia Nova Scotia British Columbia Nova Scotia California Newfoundland New Zealand British Columbia Australia California Nova Scotia Nova Scotia Australia Norway Canary Islands Mediterranean Mediterranean Canary Islands Mediterranean Canary Islands Canary Islands Experiment Observational Manipulation Manipulation Observational Manipulation Manipulation Observational Manipulation Manipulation Manipulation Observational Observational Manipulation Manipulation Observational Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Observational Observational Observational Manipulation Observational Manipulation Observational Observational Observational Manipulation Manipulation Manipulation Observational Manipulation Observational Manipulation Observational Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Manipulation Regime shift direction Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Fwd Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Rev Urchin Urchin biomass biomass Start (g.m-2) End (g.m-2) 0 12,228 0 7,767 932 6,136 5,770 5,770 0 3,107 0 2,535 1,246 1,246 1,162 1,162 0 1,014 0 883 691 691 667 667 581 581 0 507 233 233 27 210 9 150 804 0 3,506 0 464 0 2,261 0 1,057 0 559 0 401 0 757 0 456 0 2,535 0 1,521 0 1,014 0 912 0 912 0 2,900 0 4,579 0 2,317 0 4,673 0 1208 0 191 0 480 5 2,198 15 201 18 1,254 25 413 41 635 70 2,314 76 581 79 623 89 2,200 102 128 128 1,521 254 1,388 324 112 0 208 5 231 5 185 12 336 19 170 20 150 57 Urchin density end (individ. m-2) 280 90 80 322 40 10 7 4 4 34 120 93 2 2 32 7 8 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.5 0.1 15 0.1 3 0.5 0.3 0.5 0.5 13 24 1 6 0 0.2 0.2 0.5 1 1 2 % Cover canopy macroalgae start 100 55 60 39 68 95 38 71 95 98 20 84 71 95 79 54 85 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.3 0 0 0 2 0 0 0 0 0 0 0 17 0 2 0 0 0 0 % Cover canopy macroalgae end 0 7 0 1 37 20 8 12 70 0 0 0 23 70 0 12 0 59 100 100 12 93 78 37 92 91 48 30 28 28 42 100 100 88 100 100 52 100 100 25 22 9 50 100 25 100 26 90 30 95 46 41 54 54 70 85 54 % Cover Duration canopy of macroalgae response loss/gain (months) 100 3 48 7 60 4 38 11 30 7 75 5 30 3 58 6 25 5 98 3 20 24 83 1 47 6 25 5 78 3 42 6 85 9 59 4 100 12 100 6 12 10 93 18 77 18 37 18 92 18 91 18 48 18 30 18 28 5 27 18 42 18 100 72 100 12 88 20 100 25 100 36 52 13 100 33 100 12 25 21 22 8 9 10 48 300* 100 24 25 12 100 12 26 23 90 19 30 18 95 36 29 12 41 16 52 18 54 6 70 48 85 10 54 6 Source Witman, 1985 Wright et al 2005 Wright et al 2005 Foreman 1977 Wright et al 2005 Hill et al 2003 Dean et al 1984 Strain & Johnson 2009 Hill et al 2003 Johnson & Mann 1990 Miller 1985 Scheilbling et al 1999 Strain & Johnson 2009 Hill et al 2003 Scheilbling et al 1999 Hernández et al. unpub. data Hernández et al. unpub. data Johnson & Mann 1990 Duggins 1980 Kriegisch et al. unpub. data Shears & Babcock 2002 Ling 2008 Ling 2008 Ling 2008 Ling unpub. 2008-2011 Ling unpub. 2008-2011 Andrew & Underwood 1993 Andrew et al 1998 Hill et al 2003 Andrew & Choat 1982 Andrew & Choat 1982 Watson & Estes 2011 Watson & Estes 2011 Andrew 1991 Witman 1987 Scheibling, 1986 Strain & Johnson 2013 Miller & Colodey 1983 Watson & Estes 2011 Miller 1985 Cowen et al 1982 Keats et al 1990 Shears & Babcock 2003 Watson & Estes 2011 Ling et al 2010 Pearse & Hines 1979 Himmelman et al 1983 Schielbling et al 1999 Andrew et al 1998 Leinass & Christie 1996 Ortega-Borges et al 2009 Bendetti-cecchi et al 1998 Bulleri et al 1999 Hernández et al. unpub. data Hereu 2004 Hernández et al. unpub. data Hernández et al. unpub. data *Note that for purposes of calculating an average time for macroalgae recovery once urchin abundance falls below the critical reverse-shift threshold, the long-term recovery of macroalgae observed inside the Leigh Marine Reserve over 25 years (300 months) was excluded given the long lag times involved in the re-establishment of functional urchin predators and ultimately reduction in urchin abundance (Shears & Babcock 2003). 2 Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing Fig. S1. Example images of alternative macroalgal bed and grazed sea urchin barren states of rocky reef systems worldwide. Red arrows indicate the forward-shift caused by sea urchin overgrazing from macroalgal dominated to urchin dominated barrens; blue arrows indicate the reverse-shift from urchin barrens back to macroalgal habitat once grazing pressure is alleviated. Dominant macroalgal and barrens-forming sea urchin species are listed respectively - photographic credits are given in square brackets: Northern Hemisphere systems, a.) Nova Scotia (Saccharina latissima & Strogylocentrotus droebachiensis [by Scott Ling]), b.) British Columbia (Nereocystis luetkeana & S. franciscanus [by Mark Wunsch], c.) California (Pterygophora californica [by Alejandro Perez-Matus] & S. franciscanus plus S. purpuratus [by Scott Ling], d) Japan (Saccharina japonica & S. nudus [by Daisuke Fujita]), e) Mediterranean (Cystoseira balearica & Paracentrotus lividus [by Bernat Hereu]), f) Canary Islands (Lobophora variegata & Diadema africanum [by Scott Ling]); Southern Hemisphere systems, g) Australia (Ecklonia radiata & Heliocidaris erythrogramma [by Scott Ling] - for images of Australian urchin barrens formed by Centrostephanus rodgersii - see Ling & Johnson 2012; Ling 2013), h) New Zealand (E. radiata & Evechinus chloriticus [by Nick Shears], i) Chile (Lessonia trabeculata & Tetrapygus niger [by Alejandro Perez-Matus]), j) South Africa, Ecklonia maxima and Laminaria pallida [by Rob Tarr] & Parechinus angulosus [by Rob Tarr]). 3 Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing Fig. S2. Map showing the 13 globally representative temperate rocky reef systems known to occur as algal bed or urchin barrens states (temperate zones encapsulated by dotted lines within each hemisphere; equator is shown as dash-dot line). Ordered west to east from Northern to Southern Hemispheres, the representative reef systems covered 11 regions: 1. British Columbia (Gulf of Alaska, NE Pacific); 2. California (NE Pacific); 3. Nova Scotia (NW Atlantic); 4. Gulf of St. Lawrence (NW Atlantic); 5. Canary Is. (NE Atlantic); 6. Norway (Norwegian Sea, NE Atlantic); 7. Mediterranean (Mediterranean Sea, NE Atlantic); 8. Japan (NW Pacific); 9. Chile (SE Pacific); 10. South Africa (W Indian); 11. Victoria (SE Australia, SW Pacific); 12. Tasmania (SE Australia, SW Pacific); 13. New Zealand (SW Pacific Ocean). Refer to Fig. S1 for particular sea urchin and macroalgal species occurring within each reef system. 4 Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing Supplementary References Table 1 S1. S2. S3. S4. S5. S6. S7. S8. S9. S10. S11. S12. S13. S14. S15. S16. S17. S18. S19. S20. Estes, J.A. and D.O. Duggins, Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecological Monographs, 1995. 65(1): p. 75-100. Shears, N.T. and R.C. Babcock, Continuing trophic cascade effects after 25 years of no take marine reserve protection. Marine Ecology Progress Series, 2003. 246: p. 1-16. Pederson, H.G. and C.R. Johnson, Predation of the sea urchin< i> Heliocidaris erythrogramma</i> by rock lobsters (< i> Jasus edwardsii</i>) in no-take marine reserves. Journal of Experimental Marine Biology and Ecology, 2006. 336(1): p. 120-134. Ling, S., et al., Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proceedings of the National Academy of Sciences, 2009. 106(52): p. 22341-22345. Clemente, S., et al., Identifying keystone predators and the importance of preserving functional diversity in sublittoral rocky-bottom areas. Marine Ecology Progress Series, 2010. 413: p. 55-67. Ling, S. and C. Johnson, Marine reserves reduce risk of climate-driven phase shift by reinstating size-and habitat-specific trophic interactions. Ecological Applications, 2012. 22(4): p. 1232-1245. Bonaviri, C., et al., Micropredation on sea urchins as a potential stabilizing process for rocky reefs. Journal of Sea Research, 2012. 73: p. 18-23. Estes, J.A., et al., Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science, 1998. 282: p. 473-476. Steneck, R.S. Fisheries-induced biological changes to the structure and function of the Gulf of Maine ecosystem. in Proceedings of the Gulf of Maine ecosystem dynamics scientific symposium and workshop. 1997. Regional Association for Research on the Gulf of Maine Hanover, NH. Britton-Simmons, K.H., G. Foley, and D. Okamoto, Spatial subsidy in the subtidal zone: utilization of drift algae by a deep subtidal sea urchin. Aquat Biol, 2009. 5(3): p. 233-243. Kelly, J.R., K.A. Krumhansl, and R.E. Scheibling, Drift algal subsidies to sea urchins in low-productivity habitats. Marine Ecology Progress Series, 2012. 452: p. 145-157. Connell, S.D. and A.D. Irving, Integrating ecology with biogeography using landscape characteristics: a case study of subtidal habitat across continental Australia. Journal of Biogeography, 2008. 35(9): p. 1608-1621. Keats, D., G. South, and D. Steele, The effects of an experimental reduction in grazing by green sea urchins on a benthic macroalgal community in eastern Newfoundland. Marine Ecology Progress Series, 1990. 68: p. 181-193. Kawamata, S., Inhibitory effects of wave action on destructive grazing by sea urchins: a review. Bulletin of Fisheries Research Agency, 2010 32(95-102). Konar, B., Limited effects of a keystone species: trends of sea otters and kelp forests at the Semichi Islands, Alaska. Marine Ecology Progress Series, 2000. 199: p. 271-280. Kelly, J.R., R.E. Scheibling, and T. Balch, Invasion-mediated shifts in the macrobenthic assemblage of a rocky subtidal ecosystem. Mar Ecol Prog Ser, 2011. 437: p. 69-78. Dayton, P.K., et al., Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecological Applications [Ecol. Appl.]. 1998. 8(2): p. 309-322. Hart, M.W. and R.E. Scheibling, Heat waves, baby booms, and the destruction of kelp beds by sea urchins. Marine Biology, 1988. 99: p. 167-176. Sivertsen, K. Overgrazing of kelp beds along the coast of Norway. in Eighteenth International Seaweed Symposium. 2007. Springer. Tegner, M.J., et al., Sea urchin cavitation of giant kelp (Macrocystis pyriferaC. Agardh) holdfasts and its effects on kelp mortality across a large California forest. Journal of Experimental Marine Biology and Ecology, 1995. 191: p. 83-99. 5 Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing S21. S22. S23. S24. S25. S26. S27. S28. S29. S30. S31. S32. S33. S34. S35. S36. S37. S38. S39. S40. S41. Harrold, C. and D.C. Reed, Food availability, sea urchin grazing and kelp forest community structure. Ecology, 1985. 66(4): p. 1160-1169. Ling, S., et al., Reproductive potential of a marine ecosystem engineer at the edge of a newly expanded range. Global Change Biology, 2008. 14(4): p. 907-915. Johnson, C.R. and K.H. Mann, The importance of plant defence abilities to the structure of subtidal seaweed communities: The kelp< i> Laminaria longicruris</i> de la Pylaie survives grazing by the snail< i> Lacuna vincta</i>(Montagu) at high population densities. Journal of Experimental Marine Biology and Ecology, 1986. 97(3): p. 231-267. Fletcher, W.J., Interactions among subtidal Australian sea urchins, gastropods, and algae: effects of experimental removals. Ecological Monographs, 1987. 57(1): p. 89-109. Clemente, S., J. Hernández, and A. Brito, Context-dependent effects of marine protected areas on predatory interactions. Mar Ecol Prog Ser, 2011. 437: p. 119-133. Watson, J. and J.A. Estes, Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecological Monographs, 2011. 81(2): p. 215-239. Sangil, C., et al., No-take areas as an effective tool to restore urchin barrens on subtropical rocky reefs. Estuarine, Coastal and Shelf Science, 2012. 112: p. 207-215. Jones, N. and J. Kain, Subtidal algal colonization following the removal of Echinus. Helgoländ Wiss Meer, 1967. 15: p. 460-466. Lauzon-Guay, J.-S. and R.E. Scheibling, Seasonal variation in movement, aggregation and destructive grazing of the green sea urchin (Strongylocentrotus droebachiensis) in relation to wave action and sea temperature. Marine Biology, 2007. 151(6): p. 2109-2118. Gagnon, P., J. Himmelman, and L. Johnson, Temporal variation in community interfaces: kelp-bed boundary dynamics adjacent to persistent urchin barrens. Marine Biology, 2004. 144(6): p. 1191-1203. Tegner, M. and P. Dayton, Sea urchins, El Ninos, and the long term stability of Southern California kelp forest communities. Marine ecology progress series. Oldendorf, 1991. 77(1): p. 49-63. Lang, C. and K. Mann, Changes in sea urchin populations after the destruction of kelp beds. Marine Biology, 1976. 36(4): p. 321-326. Hernández, J.C., et al., Effect of temperature on settlement and postsettlement survival in a barrensforming sea urchin. Marine Ecology Progress Series, 2010. 413(6). Byrne, M., et al., Reproduction in the diadematoid sea urchin Centrostephanus rodgersii in contrasting habitats along the coast of New South Wales, Australia. Marine Biology, 1998. 132: p. 305-318. Eurich, J., R. Selden, and R. Warner, California spiny lobster preference for urchins from kelp forests: implications for urchin barren persistence. MARINE ECOLOGY PROGRESS SERIES, 2014. 498: p. 217-225. Tegner, M.J. and P.K. Dayton, Sea urchin recruitment patterns and implications of commerical fishing. Science, 1976: p. 324-326. Tegner, M.J. and P.K. Dayton, Population structure, recruitment and mortality of two sea urchins (Stronglyocentrotus franciscanus and S. purpuratus) in a kelp forest. Marine Ecology Progress Series, 1981. 5: p. 255-268. Zhang, Z., et al., Recruitment patterns and juvenile–adult associations of red sea urchins in three areas of British Columbia. Fisheries Research, 2011. 109(2): p. 276-284. Hereu, B., et al., Multiple processes regulate long-term population dynamics of sea urchins on Mediterranean rocky reefs. PloS one, 2012. 7(5): p. e36901. Scheibling, R.E. and A.W. Hennigar, Recurrent outbreaks of disease in sea urchins Stronglyocentrotus droebachiensis in Nova Scotia: evidence for a link with large-scale meteorologic and ceanographic events. Marine Ecology Progress Series, 1997. 152: p. 155-165. Pearse, J. and A. Hines, Expansion of a central California kelp forest following the mass mortality of sea urchins. Marine Biology, 1979. 51: p. 83-91. 6 Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing S42. S43. S44. S45. S46. S47. Scheibling, R.E., A.W. Hennigar, and T. Balch, Destructive grazing, epiphytism, and disease: the dynamics of sea urchin-kelp interactions in Nova Scotia. Canadian Journal of Fisheries and Aquatic Sciences, 1999. 56(12): p. 2300-2314. Scheibling, R.E. and J.-S. Lauzon-Guay, Killer storms: North Atlantic hurricanes and disease outbreaks in sea urchins. Limnology and Oceanography, 2010. 55(6): p. 2331-2338. Andrew, N.L., Changes in subtidal habitat following mass mortality of sea urchins in Botany Bay, New South Wales. Australian Journal of Ecology, 1991. 16: p. 353-362. Scheibling, R., Feehan CJ, and L.-G. JS, Climate change, disease and the dynamics of a kelp-bed ecosystem in Nova Scotia, in Climate Change: past, present and future perspectives, a global synthesis from the Atlantic. Servicio de Publicaciones de la Universidad de La Laguna, Tenerife, FernándezPalocios JM, et al., Editors. 2013. p. 41−81. Fagerli, C.W., K.M. Norderhaug, and H.C. Christie, Lack of sea urchin settlement may explain kelp forest recovery in overgrazed areas in Norway. Marine Ecology Progress Series, 2013. 488: p. 119-132. Fagerli CW, et al., Predators of the destructive sea urchin grazer Strongylocentrotus droebachiensis on the Norwegian coast Marine Ecology Progress Series, 2014. 502: p. 207-218. Supplementary References Table S2 Andrew NL (1991) Changes in subtidal habitat following mass mortality of sea urchins in Botany Bay, New South Wales. Australian Journal of Ecology 16: 353-362 Andrew NL, Choat JH (1982) The influence of predation and conspecific adults on the abundance of juvenile Evechinus chloroticus (Echinoidea: Echinometridae). Oecologia 54: 80-87 Andrew NL, Underwood AJ (1993) Density-dependent foraging in the sea urchin Centrostephanus rodgersii on shallow subtidal reefs in New South Wales, Australia. Marine Ecology Progress Series 99: 89-98 Andrew NL, Worthington DG, Brett PA, Bentley N, Chick RC, Blount C (1998) Interactions between the abalone fishery and sea urchins in New South Wales. in FRDC Final Report, ed. 1993/102 PN Benedetti-Cecchi, L, Bulleri, F, Cinelli, F. (1998) Density dependent foraging of sea urchins in shallow subtidal reefs on the west coast of Italy (western Mediterranean). Marine Ecology Progress Series 163: 203-211 Bulleri F, Benedetti-Cecchi L, Cinelli F (1999) Grazing by the sea urchins Arbacia lixula L. and Paracentrotus lividus Lam. in the Northwest Mediterranean. Journal of Experimental Marine Biology and Ecology 241: 81-95 Cowen, Robert K., Catherine R. Agegian, Foster MS (1982) The maintenance of community structure in a central California giant kelp forest. Journal of Experimental Marine Biology and Ecology 64: 189-201 Dean TA, Schroeter SC, Dixon JD (1984) Effects of grazing by two species of sea urchins (Strongylocentrotusfranciscanus and Lytechinus anamesus) on recruitment and survival of two species of kelp (Macrocystis pyrifera and Pterygophora californica). Marine Biology 78, 301-313 Duggins DO (1980) Kelp beds and sea otters: An experimental approach. Ecology 61:447-453 Foreman RE (1977) Benthic community modification and recovery following intensive grazing by Strongylocentrotus droebachiensis. Helgoland Wiss Meer 30:468-484 Hereu, B (2004) The role of trophic interactions between fishes, sea urchins and algae in the northwest Meditterannean rocky infralittotal. PhD Thesis, Department d’Ecologia, Universitat de Barcelona. Hill NA, Blount C, Poore AGB, Worthington D, Steinberg PD (2003) Grazing effects of the sea urchin Centrostephanus rodgersii in two contrasting rocky reef habitats: effects of urchin density and its implications for the fishery. Marine & Freshwater Research 54: 691-700 Himmelman JH, Cardinal A, Bourget E (1983) Community development following removal of urchins, Strongylocentrotus droebachiensis, from the rocky subtidal zone of the St. Lawrence Estuary, Eastern Canada. Oecologia 59:27-39 Johnson CR, Mann KH (1993) Rapid succession in subtidal understorey seaweeds during recovery from overgrazing by sea urchins in eastern Canada. Botanica Marina 36: 63-77 Keats DW, South GR, Steele DH (1990) The effects of an experimental reduction in grazing by green sea urchins on a benthic macroalgal community in eastern Newfoundland. Marine Ecology Progress Series 68:181-193 7 Electronic Supplementary Material: S.D. Ling et al. Global regime shift dynamics of catastrophic sea urchin overgrazing Leinaas HP, Christie H (1996) Effects of removing sea urchins (Strongylocentrotus droebachiensis): stability of the barren state and succession of kelp forest recovery in the east Atlantic. Oecologia 105:524-536 Ling SD (2008) Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156:883-894 Ling SD, Ibbott S, Sanderson JC (2010) Recovery of canopy-forming macroalgae following removal of the enigmatic grazing sea urchin Heliocidaris erythrogramma. Journal of Experimental Marine Biology and Ecology 395:135-146 Miller RJ (1985) Succession in sea urchin and seaweed abundance in Nova Scotia, Canada. Mar Biol 84:275-286 Miller RJ, Colodey AG (1983) Widespread mass mortalities of the green sea urchin in Nova Scotia, Canada. Marine Biology 73:263-267 Ortega-Borges L, Tuya F, Haroun RJ (2009) Does depth and sedimentation interact with sea urchins to affect algal assemblage patterns on eastern Atlantic reefs? Journal of Shellfish Research 28: 947-955 Pearse JS, Hines AH (1979) Expansion of a central California kelp forest following the mass mortality of sea urchins. Marine Biology 51: 83-91 Scheibling R (1986) Increased macroalgal abundance following mass mortalities of sea urchins (Strongylocentrotus droebachiensis) along the Atlantic coast of Nova Scotia. Oecologia 68:186-198 Scheibling RE, Hennigar AW, Balch T (1999) Destructive grazing, epiphytism, and disease: the dynamics of sea urchinkelp interactions in Nova Scotia. Canadian Journal of Fisheries and Aquatic Sciences 56: 2300-2314 Shears NT, Babcock RC (2002) Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132:131-142 Shears NT, RC Babcock (2003) Continuing trophic cascade effects after 25 years of no-take marine reserve protection. Marine Ecology Progress Series 246:1-16 Strain EM, Johnson CR (2009) Competition between an invasive urchin and commercially fished abalone: effect on body condition, reproduction and survivorship. Marine Ecology Progress Series 377:169-182 Strain EM, Johnson CR (2013) The effects of an invasive habitat modifier on the biotic interactions between two native herbivorous species and benthic habitat in a subtidal rocky reef ecosystem. Biological Invasions 15:1391–1405 Watson J, Estes JA (2011) Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecological Monographs 81:215-239 Witman JD (1985) Refuges, biological disturbance, and rocky subtidal community structure in New England. Ecological monographs 55: 421-445 Witman JD (1987) Subtidal coexistence: storms, grazing, mutualism, and the zonation of kelps and mussels. Ecological Monographs 57: 167-187 Wright JT, Dworjanyn SA, Rogers CN, Steinberg PD, Williamson JE, Poore, AG (2005) Density-dependent sea urchin grazing: differential removal of species, changes in community composition and alternative community states. Marine Ecology Progress Series 298: 143-156 8






