PowerPoint-Präsentation
advertisement

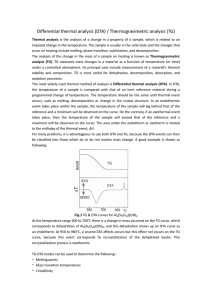
Practical aspects of thermodynamic analysis Dynamic methods of phase equilibrium studies – DTA, HF-DSC a. Unary system; b. Binary and ternary systems c. TGA; d. heat capacity measurements using DSC Isothermal methods (Phase equilibria, Diffusion couple); Influence of kinetics Applications 1. 2. 3. 4. Classification of experimental methods DTA/DSC for unary systems Temperature and enthalpy calibration Problems Phase diagrams: relations to microstructure and properties Eutectic phase diagram Phase diagram with peritectic Experimental methods used for phase diagram construction Dynamic methods Static methods Metals Ceramics Arc-melting Induction melting Solid state reactions Co-precipitation and pyrolysis Homogenisation DTA/TGA DSC Dilatometry HMA Heat treatment: Natural cooling or quenching Quenching or in-situ study Diffusion couples Charactirisation methods XRD – phase identification, phase boundary determination SEM/EDX microstructure examination, phase composition determination (for identification) EPMA for more precise phase identification EBSD for structure determination Thermal Analysis: Dynamic Methods Measurement of physical property of a substance as a function of temperature using controlled temperature program Method Measured property Application Differential Thermal Analysis (DTA) Temperature difference Phase reactions, phase transformations Differential Scanning Calorimetry (DSC) Heat flow Specific heat, Heat of transition Thermal Gravimetric Analysis (TGA) Mass Change Reactions with the gas phase, decomposition reactions Dilatometry Size Change Phase transformations, Thermal expansion Thermomechanical Analysis (TMA) Mechanical properties Materials testing DTA/Heat-Flux DSC The temperature is not measured in the sample, but at the bottom of crucible. Temperature calibration is necessary. DTA/HF-DSC signal is difference between sample and reference thermocouples. It is usually given in mV. For some devices it is given as temperature difference; this means that reference table or equation was used by instrument to calculate temperature from voltage difference. The DTA Signal Heat is transferred between furnace, crucible, sample (reference) and thermocouple 𝐴𝜆 Steady –state condition ∆Φ𝑆𝑅 = − Δ𝑙 Δ𝑇𝑆𝑅 = −𝐾Δ𝑇 DFSR – difference in heat flow rate, l – thermal conductivity TW is furnace temperature, TC is temperature of crucible TT is temperature of thermocouple, TS is sample temperature Idealized curve Real curve DTA signal Fr=L(Tm-TS) L is apparent thermal conductance to the sample, Tm is measured temperature, TS is sample temperature 𝑑Φ𝑟 𝑑𝑇𝑚 𝑑𝑇𝑆 =𝐿 − 𝑑𝑡 𝑑𝑡 𝑑𝑡 ≈b =0 b is heating rate 𝑑Φ𝑟 𝑑𝑇𝑚 =𝐿 𝑑𝑡 𝑑𝑡 𝑑Φ𝑟 𝑑𝑡 𝑑Φ𝑟 = =𝐿 𝑑𝑡 𝑑𝑇𝑚 𝑑𝑇𝑚 DTA signal for unary system DTA responses to melting and freezing of pure substance: a- onset temperature, b- peak at temperature c . Due to dynamic character of experiment, the temperature distribution is never completely homogeneous. The temperature is not measured in the sample, but at the bottom of crucible. That is why temperature correction is necessary. Temperature calibration Measured temperature vs. time and associated DTA plot for melting of pure Sn. Black is measurement with instrument thermocouple, red is results from a thermocouple immersed directly in the Sn sample. Temperature calibration: establishment of relation between measured temperature Tmeas indicated by the instrument and the true temperature Ttr. At least three substances (usually pure metals) with melting temperature covering temperature range of interest should be selected. Mass should be corresponded to recommended mass for measurement in this instrument. Measurement should be done with different heating range b and extrapolated to b=0. Temperature calibration Measured temperature of thermal event depends on mass and cooling rate b. Extrapolated T melting of Sn to b=0 for different sample mass Substance T, °C Sn 231.928 Al 660.323 Ag 961.78 Au 1064.18 Pd 1554.8 Temperature correction for Ga, In and Sn Plotting DTA signal vs. Temperature or time DTA melting of pure Ag at 10 K/min Reference thermocouple Ttcref and sample thermocouple Ttcsample vs. time DT=Ts-Tref is difference between sample temperature and reference Red line – DT vs. time Solid black line - DT vs. Ts Dashed line – DT vs. Tref Tref=T0+bT DT vs. time is necessary for quantitative determination of enthalpy. DT vs T sample is better for determination of temperature of thermal event. Enthalpy calibration KH is instrument sensitivity for Ga, In, Sn 𝑡2 𝑚𝑠𝑎𝑚𝑝𝑙𝑒 ∆𝐻𝑠𝑎𝑚𝑝𝑙𝑒 = 𝐾𝐻 ∆𝑇 𝑡 𝑑𝑡 𝑡1 Recommended values of temperatures and enthalpies of melting of metals Element Tmelt (°C) DHm(J/g) Ga 29.764 80.07 In 156.598 28.62 Sn 231.928 60.38 Zn 419.527 108.09 Al 660.323 399.87 Ag 961.78 104.61 Au 1064.18 64.58 Enthalpy calibration factors for each calibration substance are represented as a function of transition temperature. Provided the dependence on heating rate b and sample mass are negligible (within scatter of individual experiments) the enthalpy calibration factor KH(Ttr, m, b) give the enthalpy calibration function KH(T). Problems Influence of mass and heating rate: Larger mass and larger heating rate produce larger peak, but make detection of closely spaced thermal events more difficult. Blue 5 K/min, red-10 K/min, black – 15 K/min Problems Sample shape is typically not conform to the shape of the sample cup. The thermal contact area between the sample and crucible will change during melting process. Possible solution is second heating. However in case of complicated phase diagram and not equilibrium freezing different phase assemblage can be present in the sample before second heating. Undercooling problem with liquidus determination on cooling Measured temperature vs. Time (a) and DTA signal vs. Temperature for freezing of pure Sn. The instrument thermocouple readings are black and from thermocouple immersed directly into Sn sample are red. For the immersed thermocouple the temperature reheats up to melting temperature as heat of fusion is released rapidly. Many metals and alloys are prone to undercooling before the nucleation of solid phase start from melt. Nucleation temperature can differ from liquidus up to 100 or more degree depending on nature of alloy system and other factors. Determination of melting on heating is more reliable. Problems Powder sample increase oxidation, reduce heat flaw Solutions: Inert powder cover – to increase the thermal conduction to the sample cup Lid – to reduce material loss and contamination of the instrument, to prevent sample radiation loss and maintain an isothermal sample Atmosphere. Commercial purity inert gas is no adequate. Use of high purity inert gas to a Ti getter is recommended. Helium (He) has higher thermal conductivity than Ar; the choice can alter thermal transport rates in DTA instrument. Calibration should be performed with the same gas as used for samples Crucible selection/reaction. High purity Al2O3 is standard DTA crucibles for metals investigation. Use of ZrO2 and Y2O3 crucibles can be recommended for highly reactive metals. Carbon crucibles can be recommended for metals not forming carbides. Pt, W crucibles can be used for ceramic materials Good praxis for DTA experiments Calibration: Based on the melting point of pure substances. Crucible, standard material, heating rate, sample mass, atmosphere are kept constant Characterisation: The composition of samples and crystal structure have to be investigated before and after the measurement Combination: DTA experiments tell us that something is happening at a specific temperature. They usually do not tell us, what is happening. Combination with other methods like X-ray diffraction, spectroscopy, microscopic investigation and composition analysis (e.g. Electron probe microanalysis) are required to interpret the results DTA vs. DSC DTA DSC Heat-flux Power compensation DT DT between sample and reference T and DH of transformation DT between sample and reference More robust, measurements can be done in wider T range, in more aggressive environment (oxidation atmosphere), possible combination with TGA to measure mass change T and DH of transformation, Cp measurements HF-DSC is more sensitive than DTA, possible to measure heat capacity Power compensation to keep the same temperature in both furnaces T and DH of transformation, Cp measurements More effective, since response time is shorter than in HF-DSC