Dosyayı İndir
advertisement

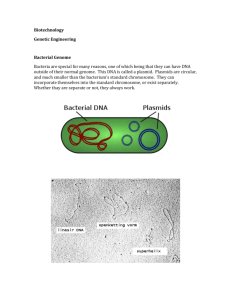
Chapter 27 Genetic engineering of plants Terms to know Transgene: It is a gene or genetic material that has been transferred naturally or by any of a number of genetic engineering techniques from one organism to another. Transgesis : The process of introducing an exogenous gene called a transgene into a living organisms so that the organism will exhibit a new property and transmit that property to its offspring. Transgenic Plants : The plants which expresses the characters coded by the transgene are called Transgenic plants. History of Plant Breeding Selective Breeding used in the History Genetics studies started with Mendel Cross pollination : Pollen from one plant to stigma of another plant. Found dominate characteristics in plants Uses of Traditional Breeding: Increase crop yield Increase Resistance to pests and diseases Drought tolerance Disadvantages of Traditional breeding: Long process Lot of man power Limited possibility of improved traits. The Reproductive Organs of a Typical Plant : Pollen grains are the male reproductive cells of the plant. They are made in the anther (orange), the top portion of the stamen. The female reproductive cells, the ova, are sequestered in the ovary. Pollen reaches the ova via the stigma, which is attached to the ovary by the pistil. Mutation Breeding Treat seeds with mutagens or expose to X rays or gamma rays. Disadvantages Less predictable results Lot of man power Successful in the flower world. Eg; new colours, more petals. UV Treatment or Mutagens Seeds Killed Alive Planted Tested for Improvements Found desirable traits Test for Progeny heritable Sold to Markets Transgenic plant : Insertion of a foreign gene into a specific plant. Difference between Trangenic Technology and traditional Breeding: Trangenic Technology : Transform gene from any source. Eg: animals, bacteria, virus etc Traditional Breeding : Move genes only between members of a particular genus of plants. Plant Tissue Culture Totipotency : Ability of a cell to divide into any type of cell. Explant : Mass of tissue or cells Solid medium – Callus culture. Tissue can be immature embryo, apical meristem, root tip Liquid medium – suspension culture Tissue should be protoplast (cells with no cell wall), micro or macrospores. Nutrients and hormones are used for growth and development. Eg : 2,4 dichlorophenoxyacetic acid (analogous to auxin) Callus : Undifferentiated cell which form a crystalline white layer on solid medium. I. Move callus to other medium with reduced hormones which allows shoot to develop. II. Move the callus to other medium with no hormone which allows root hairs to grow. The process of regenerating a plant from a single cell may cause three types of alterations, 1. Temporary Physiological change 2. Epigenetic change 3. True genetic changes An Entire Plant Can Be Regenerated from a Single Cell Small samples of tissue, or even single plant cells may be cultured in vitro. Under appropriate conditions, these may regenerate into complete plants. FIGURE 14.3 Callus or Liquid Culture of Plant Cells Can Regenerate Entire Plants In callus culture a mass of undifferentiated cells grows on a solid surface. In liquid culture, separated single cells are grown. Both types of cultures can develop shoots and roots with appropriate manipulation of plant hormone levels. Gene transfer in plants Why gene transfer? • • • • • Crop improvement Disease resistance Stress tolerance Improved performance Value-added traits Basic studies • Gene expression • Reverse genetics - understanding functioning of unknown genes • Biochemistry and metabolism Gene transfer strategies: Systems and vectors • Agro bacterium • Direct DNA uptake • Virus-based vectors Plant transformatIon wIth the Ti plasmid of AgrobacterIum tumefacIens A. tumefaciens is a gram-negative soil bacterium which naturally transforms plant cells, resulting in crown gall (cancer) tumors Tumor formation is the result of the transfer, integration and expression of genes on a specific segment of A. tumefaciens plasmid DNA called the T-DNA (transferred DNA) The T-DNA resides on a large plasmid called the Ti (tumor inducing) plasmid found in A.tumefaciens Agrobacterium-mediated gene transfer The keys • To make a segment of DNA that contains a selectable marker and a gene of interest to look like a T-DNA • To get this “T-DNA” into an Agrobacterium cell so that it can be mobilized by the vir genes • To produce and find transformed plant cells that can be regenerated into normal, fertile plants Requirements • A transfer cassette bounded by functioning borders • Ways to get this cassette into Agrobacterium • Disarmed Ti plasmids that retain functional vir genes Advantages • Technically simple • Yields relatively uncomplicated insertion events (low copy number, minimal rearrangements) • Unlimited size of foreign DNA • Efficient (for most plants) • Adaptable to different cell types, culture procedures (protoplasts, tissue sections, “non-culture” methods) • Transformants are mitotically and meiotically stable Disadvantages • Host range is limited: not all plants may be susceptible to Agrobacterium • With susceptible plants, accessible culture/regeneration systems must be adaptable to Agrobacterium-mediated gene transfer The Infection process Wounded plant cell releases phenolics and nutrients. Phenolics and nutrients cause chemotaxic response of A. tumefaciens Attachment of the bacteria to the plant cell. Certain phenolics (e.g., acetosyringone, hydroxyacetosyringone) induce vir gene transcription and allow for T-DNA transfer and integration into plant chromosomal DNA. Transcription and translation of the T-DNA in the plant cell to produce opines (food) and tumors (housing) for the bacteria. The opine permease/catabolism genes on the Ti plasmid allow A. tumefaciens to use opines as a C, H, O, and N source. FIGURE 14.4 Agrobacterium Transfers Plasmid DNA into Infected Plants Agrobacterium carrying a Ti plasmid is attracted by acetosyringone to a wounded plant stem. The Ti plasmid is cut by endonucleases to release single-stranded T-DNA, which is covered with protective proteins, and transported into the plant cell through a conjugation-like mechanism. The T-DNA enters the plant nucleus where it integrates into plant chromosomal DNA. The Ti plasmıd of Agrobacterıum tumafacıens and the transfer of ıts T-DNA to the plant nuclear genome Crown Gall on Tobacco Infectıon of a plant wıth A. tumefacıens and formatıon of crown galls Clone YFG (your favorite gene) or the target gene in the small T-DNA plasmid in E. coli, isolate the plasmid and use it to transform A. tumefaciens containing the disarmed Ti plasmid Essential Elements for Carrying a Transgene on Ti Plasmids The T-DNA segment contains both a transgene and a selective marker or reporter gene. These have separate promoters and termination signals. The marker or reporter gene must be expressed all the time, whereas the transgene is often expressed only in certain tissues or under certain circumstances and usually has a promoter that can be induced by appropriate signals. Ti plasmid structure & function FIGURE 14.6 Transfer of Modified Ti Plasmid into a Plant Agrobacterium carrying a Ti plasmid is added to plant tissue growing in culture. The T-DNA carries an antibiotic resistance gene (neomycin in this figure) to allow selection of successfully transformed plant cells. Both callus cultures (A) and liquid cultures (B) may be used in this procedure. 20 Gene Gun or Biolistic Method A gene gun is used for injecting cells with genetic information, it is also known as biolistic particle delivery system. Biotechnological Applications of Plant Breeding Genetically modified crops • All plant characteristics, such as size, texture, and sweetness, are determined on the genetic level. • • • • • • Also: The hardiness of crop plants. Their drought resistance. Rate of growth under different soil conditions. Dependence on fertilizers. Resistance to various pests and diseases. • Used to do this by selective breeding Why would we want to modify an organism? • Better crop yield, especially under harsh conditions. • Herbicide or disease resistance • Nutrition or pharmaceuticals, vaccine delivery • “In 2010, approximately 89% of soy and 69% of corn grown in the U.S. were grown from Roundup Ready® seed.” http://www.oercommons.org/courses/detecting-genetically-modified-food-by-pcr/ Roundup Ready Gene • The glyphosate resistance gene protects food plants against the broad-spectrum herbicide Glyphosate - N(phosphonomethyl) glycine [Roundup®], which efficiently kills invasive weeds in the field. • The major advantages of the "Roundup Ready®” system include better weed control, reduction of crop injury, higher yield, and lower environmental impact than traditional weed control systems. • Notably, fields treated with Roundup® require less tilling; this preserves soil fertility by lessening soil run-off and oxidation.” Glyphosate - N-(phosphonomethyl) glycine • An aminophosphonic analogue of the natural amino acid glycine. • It is absorbed through foliage and translocated to actively growing points. (Meristems!!!) Glyphosate • Mode of action is to inhibit an enzyme involved in the synthesis of the aromatic amino acids: • tyrosine, • tryptophan • phenylalanine Glycine Glyphosate - N-(phosphonomethyl) glycine • It does this by inhibiting the enzyme 5enolpyruvylshikimate-3-phosphate synthase (EPSPS), which catalyzes the reaction of shikimate-3-phosphate (S3P) and phosphoenol pyruvate to form 5enolpyruvyl-shikimate-3-phosphate (ESP). Glyphosate • ESP subsequently dephosphorylated to chorismate, an essential precursor in plants for these aromatic amino acids. Glycine Roundup Ready Gene • Glyphosate functions by occupying the binding site of the phosphoenol pyruvate, mimicking an intermediate state of the enzyme substrates complex. • The "Roundup Ready®” system introduces a stable gene alteration which prevents Glyphosate binding and allowing the formation of the essential aromatic amino acids Roundup Ready Gene • The shikimate pathway is not present in animals, which instead obtain aromatic amino acids from their diet. • Glyphosate has also been shown to inhibit other plant enzymes •Also has been found to affect animal enzymes. •The United States Environmental Protection Agency considers glyphosate to be relatively low in toxicity, and without carcinogenic or teratogenic effects •However, some farm workers have reported chemical burns and contact skin burns Environmental degradation • When glyphosate comes into contact with the soil, it can be rapidly bound to soil particles and be inactivated. • Unbound glyphosate can be degraded by bacteria. – However, glyphosate has been shown to increase the infection rate of wheat by fusarium head blight in fields that have been treated with glyphosate. • In soils, half-lives vary from as little as 3 days at a site in Texas to 141 days at a site in Iowa. • In addition, the glyphosate metabolite amino methyl phosphonic acid has been shown to persist up to 2 years in Swedish forest soils. • Glyphosate absorption varies depending on the kind of soil. Insect Resistance • B. thuringiensis (commonly known as 'Bt') is an insecticidal bacterium, marketed worldwide for control of many important plant pests - mainly caterpillars of the Lepidoptera (butterflies and moths) but also mosquito larvae, and simuliid blackflies that vector river blindness in Africa. • Bt products represent about 1% of the total ‘agrochemical’ market (fungicides, herbicides and insecticides) Genetically modified crops • 1992- The first commercially grown genetically modified food crop was a tomato - was made more resistant to rotting, by adding an anti-sense gene which interfered with the production of the enzyme polygalacturonase. – The enzyme polygalacturonase breaks down part of the plant cell wall, which is what happens when fruit begins to rot. Genetically modified crops • Need to build in a: • Promoter • Stop signal ON/OFF Switch PROMOTER INTRON Makes Protein CODING SEQUENCE stop sign poly A signal Genetically modified crops • So to modify a plant: • Need to know the DNA sequence of the gene of interest • Need to put an easily identifiable maker gene near or next to the gene of interest • Have to insert both of these into the plant nuclear genome • Good screen process to find successful insertion Building the Transgenes ON/OFF Switch PROMOTER INTRON Makes Protein CODING SEQUENCE Plant Transgene Plant Selectable Marker Gene Plasmid DNA Construct bacterial genes •antibiotic marker •replication origin stop sign poly A signal Cloning into a Plasmid • The plasmid carrying genes for antibiotic resistance, and a DNA strand, which contains the gene of interest, are both cut with the same restriction endonuclease. • The plasmid is opened up and the gene is freed from its parent DNA strand. They have complementary "sticky ends." The opened plasmid and the freed gene are mixed with DNA ligase, which reforms the two pieces as recombinant DNA. Cloning into a Plasmid • Plasmids + copies of the DNA fragment produce quantities of recombinant DNA. • This recombinant DNA stew is allowed to transform a bacterial culture, which is then exposed to antibiotics. • All the cells except those which have been encoded by the plasmid DNA recombinant are killed, leaving a cell culture containing the desired recombinant DNA. So, how do you get the DNA into the Plant? Meristems Injections • REMEMBER!!!!!!! • The tissue in most plants consisting of undifferentiated cells (meristematic cells), found in zones of the plant where growth can take place. • Meristematic cells are analogous in function to stem cells in animals, are incompletely or not differentiated, and are capable of continued cellular division. • First method of DNA transfer to a plant. • Inject DNA into the tip containing the most undifferentiated cells – more chance of DNA being incorporated in plant Genome • Worked about 1 in 10,000 times! Tunica-Corpus model of the apical meristem (growing tip). The epidermal (L1) and subepidermal (L2) layers form the outer layers called the tunica. The inner L3 layer is called the corpus. Cells in the L1 and L2 layers divide in a sideways fashion which keeps these layers distinct, while the L3 layer divides in a more random fashion. Particle Bombardment Particle Bombardment Particle-Gun Bombardment 1. DNA- or RNA-coated gold/tungsten particles are loaded into the gun and you pull the trigger. Selected DNA sticks to surface of metal pellets in a salt solution (CaCl2). Particle Bombardment 2. A low pressure helium pulse delivers the coated gold/tungsten particles into virtually any target cell or tissue. 3. The particles carry the DNA cells do not have to be removed from tissue in order to transform the cells 4. As the cells repair their injuries, they integrate their DNA into their genome, thus allowing for the host cell to transcribe and translate the transgene. Particle Bombardment The DNA sometimes was incorporated into the nuclear genome of the plant Gene has to be incorporated into cell’s DNA where it will be transcribed Also inserted gene must not break up some other necessary gene sequence Agrobacterium tumefaciens Overall process – Uses the natural infection mechanism of a plant pathogen – Agrobacterium tumefaciens naturally infects the wound sites in dicotyledonous plant causing the formation of the crown gall tumors. – Capable to transfer a particular DNA segment (T-DNA) of the tumor-inducing (Ti) plasmid into the nucleus of infected cells where it is integrated fully into the host genome and transcribed, causing the crown gall disease. • So the pathogen inserts the new DNA with great success!!! Overall process • The vir region on the plasmid inserts DNA between the Tregion into plant nuclear genome • Insert gene of interest and marker in the T-region by restriction enzymes – the pathogen will then “infect” the plant material • Works fantastically well with all dicot plant species – tomatoes, potatoes, cucumbers, etc – Does not work as well with monocot plant species - corn • As Agrobacterium tumefaciens do not naturally infect monocots Overview of the Infection Process Ti plasmids and the bacterial chromosome act in concert to transform the plant 1. Agrobacterium tumefaciens chromosomal genes: chvA, chvB, pscA required for initial binding of the bacterium to the plant cell and code for polysaccharide on bacterial cell surface. 2. Virulence region (vir) carried on pTi, but not in the transferred region (T-DNA). Genes code for proteins that prepare the T-DNA and the bacterium for transfer. 3. T-DNA encodes genes for opine synthesis and for tumor production. 4. occ (opine catabolism) genes carried on the pTi and allows the bacterium to utilize opines as nutrient. Agrobacterium chromosomal DNA pscA chvA chvB T-DNA-inserts into plant genome tra for transfer to the vir genes plant oriV pTi bacterial conjugation opine catabolism Agrobacterium tumafaciens senses Acetosyringone via a 3-component-like system 3 components: ChvE, VirA, VirG