animal development
advertisement

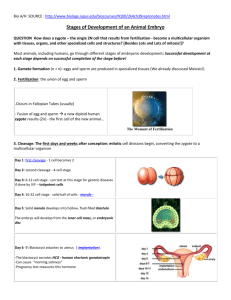
ANIMAL DEVELOPMENT CHAPTER 47 Figure 47.0 Human embryo SEA URCHIN FERTILIZATION • Acrosomal Reaction – sperm release hydrolytic enzymes that allow acrosomal process to penetrate jelly coat of egg • Membranes of sperm and egg fuse together • Causes membrane depolarization (change in membrane potential) which is the fast block to polyspermy • Cortical reaction – fusion triggers signal transduction that causes ER to release Ca2+ that in turn causes cortical granules to release their contents into space between membrane and vitelline layer • Causes osmosis and swelling of space which is the slow block to polyspermy • High Ca2+ also causes metabolic activity to begin in egg Figure 47.2 The acrosomal and cortical reactions during sea urchin fertilization Figure 47.3 A wave of Ca2+ release during the cortical reaction MAMMAL FERTILIZATION • Differences between sea urchin and mammals: – Oocyte cloaked in follicle cells – Zone pellucida is like the vitelline layer – Haploid nuclei of sperm and egg do not immediately fuse, but only after first cell division Figure 47.5 Fertilization in mammals Figure 47.4 Timeline for the fertilization of sea urchin eggs VOCABULARY • Cleavage – cell division after fertilization; embryo doesn’t grow • Yolk – where nutrients are stored; most plentiful in birds, reptiles and fish • Gastrulation – forms three layers (ectoderm, endoderm, and mesoderm) • Organogenesis – generation of rudimentary organs Figure 47.6 Cleavage in an echinoderm (sea urchin) embryo Figure 47.6x Sea urchin development, from single cell to larva CLEAVAGE • Meroblastic cleavage – incomplete cell division due to yolk • Holoblastic – complete cell division (little, moderate, or no yolk) • Most animals (except mammals) have definite polarity due to heterogeneously distributed materials (such as yolk) • Animal pole – no yolk end of embryo • Vegetal pole – yolk end of embryo • In frogs, plasma membrane and cortex rotate to form grey crescent • In birds, cleavage is meroblastic • In humans and sea urchins, holoblastic cleavage Figure 47.7 The establishment of the body axes and the first cleavage plane in an amphibian Figure 47.8x Cleavage in a frog embryo Figure 47.8d Cross section of a frog blastula GASTRULATION • Sea Urchin – Single-celled blastula wall – Mesenchyme cells migrate to form mesoderm – Invagination forms archenteron – Blastopore becomes anus Figure 47.9 Sea urchin gastrulation • Frog – More yolk and wall of blastula more than one cell thick – Invagination forms blastopore and dorsal lip – Involution – cells on outer surface rolling over lip to inside of embryo – Blastopore sealed off by yolk plug Figure 47.10 Gastrulation in a frog embryo • Bird – Primitive streak, a groove, forms rather than a blastopore (round) – Only epiblast cells contribute to embryo, while hypoblast cells direct formation of primitive streak and aid in other development Figure 47.12 Cleavage, gastrulation, and early organogenesis in a chick embryo ORGANOGENESIS • First organs formed are notochord and neural tube • Notochord – skeletal rode common to all chordates • Neural tube becomes the brain and spinal cord • Somites become vertebrate • Neural crest (only vertebrates) – cells that migrate to form a variety of things Figure 47.11 Organogenesis in a frog embryo TISSUE LAYERS • Only part of each layer contributes to actual embryo • Extraembryonic membranes support growing embryo: – Yolk sac, Amnion, Chorion, and Allantois Figure 47.14 The development of extraembryonic membranes in a chick Early development of a human embryo and its extraembryonic membranes MAMMALIAN DEVELOPMENT • Amnion – encloses embryo in fluid filled sac (“water breaks”) • Chorion – cushions embryo (outside of amnion) • Allantois – extension of hind gut; incorporated into umbilical cord (stores uric acid waste in birds) • Yolk sac - encloses fluid filled cavity or yolk (in birds etc.) MORPHOGENESIS • Involves specific changes in cell shape, position, motility, and adhesion • Developmental fate depends on cytoplasmic determinants • Fate mapping can reveal cell genealogies • Cell fate and pattern formation determined by inductive signals • Remember the ZPA and Hox gene Figure 47.16 Change in cellular shape during morphogenesis Figure 47.20 Fate maps for two chordates Figure 47.21 Experimental demonstration of the importance of cytoplasmic determinants in amphibians Figure 47.22 The “organizer” of Spemann and Mangold Figure 47.23 Organizer regions in vertebrate limb development Figure 47.24 The experimental manipulation of positional information This undated computed axial tomography (CT scan) provided on Thursday, Nov. 8, 2007, by the Sparsh Hospital shows 2-year-old Lakshmi before she underwent surgery in Bangalore, India . The Indian girl born with four arms and four legs. kidneys and other body parts of the undeveloped fetus. Lakshmi, who has been revered by some in her village as a reincarnation of the four-armed Hindu goddess she was named for, was born joined at the pelvis to a “parasitic twin” that stopped developing in her mother’s womb. The surviving fetus absorbed the limbs, Mutated Hox gene (HoxD13)