SOLUTIONS

SOLUTIONS
• Solutions : Homogeneous mixture of two or more substances.
Consist of a solute and a solvent.
• Properties of a solution
• Solutions have variable composition
• Solutions are always clear ( transparent to light)
• Solutions are homogeneous
• Solutions do not settle out
• Solutions can be separated by physical means
• Solutions pass through filter paper
SOLUBILITY (FORMATION) OF SOLUTIONS
• Ionic substances (NaCl) will dissolve in polar solvents (H
2
O)
• The positive hydrogen atoms of water attract the negative Cl
ions.
• The negative oxygen atoms of water attract the positive Na + ions.
• This results in hydration (solvation) of the Na
+ and
Cl
ions.
NaCl dissolves in water because it is ionic (polar)
Nonpolar compounds like Cl
2 and oil do not dissolve in polar solvents like water.
Like dissolves like.
Factors affecting solubilities.
* Temperature .
Solubility of solid solutes generally increases with increase in temperature. However there are exceptions, e.g. Ce(SO
4
)
3 solubility decreases.
NaCl little change.
Gases become less soluble with incr. in temperature, e.g. SO
2
.
* Pressure .
Solids and liquids are unaffected by change in pressure.
For gases solubility in liquid increases with increase in partial pressure above the liquid. ( Henry’s Law ) e.g. Soda Can.
IONIZATION
.
Some solutes ionize in solution.
Ionization results in the formation of
ions
or
electrolytes
. (Biologically referred to as minerals)
Electrolytes
are substances whose water solution conducts electricity. They dissociate into ions when placed in water (ionisation). Ions are charged and the net charge is zero.
Nonelectrolytes
do not conduct electricity.
Conductivity.
When electrolytes are placed in solution they dissociate into ions.
The ions are attracted to oppositely charged electrodes, i.e. cations attracted to cathode (- electrode) and anions attracted to anode (+ electrode). This can result flow of electricity.
ELECTROLYTES
Strong electrolytes : ionize completely in solution e.g. NaCl → Na + + Cl -
Weak electrolytes : ionize partially in solution.
e.g. Acetic acid (vinegar)
CH
3
COOH H + + CH
3
COO -
EQUIVALENTS
UNIT USED TO EXPRESS THE AMOUNT OF EACH ION IN SOLUTION.
Amount of ion in solution (e.g. body fluids) determined by equivalent (Eq).
This is the amount of each ion (positive or negative ion) in 1 mole of solution.
Relates the amount of an ion to its charge.
e.g.
NaCl → Na + + Cl -
1 mole of Na + ions has 1 equivalent (Eq) of positive charges
1 mole of Cl ions has 1 equivalent of negative charges.
MgO → Mg 2+ + O 2-
1 mole of Mg 2+ ions has 2 equivalent (Eq) of positive charges
1 mole of O 2ions has 2 equivalent of negative charges
NB: Number of positive ions always equal number negative ions in neutral solution.
How many equivalents (Eq) are present in each of the following?
a.
0.5 mol K
+
b.
0.75 mol Ca
2+
c.
0.5 mol PO
4
3-
MILLIEQUIVALENTS (mEq)
1 Eq = 1000 mEq
Used to determine concentration of ions in the body.
e.g.
How many mEq of the Calcium ion are there in 0.4 g of
CaBr
2.
e.g.
The blood Magnessium level for a patient was found to be 16.0 mEq/L. How many moles of magnessium ion are in 0.4 0 L of blood?
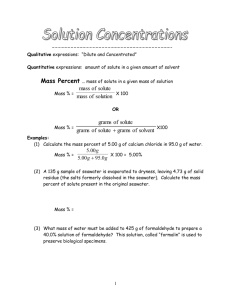
Concentration Units
Weight/Volume Percent Concentration
• The concentration of a solution tells how much solute is dissolved in a given amount of solution.
• Weight/volume percent concentration, (w/v)%, is the number of grams of solute dissolved in 100 mL of solution.
Weight/volume percent concentration
(w/v)% = mass of solute (g) volume of solution (mL) x 100%
10
Concentration Units
Weight/Volume Percent Concentration
For example, vinegar contains 5 g of acetic acid in
100 mL of solution, so the (w/v)% concentration is
5 g acetic acid
100 mL vinegar solution x 100% = 5% (w/v)
11
Concentration Units
Volume/Volume Percent Concentration
Volume/volume percent concentration
(v/v)% = volume of solute (mL) volume of solution (mL) x 100%
For example, if a bottle of rubbing alcohol contains
70 mL of 2-propanol in 100 mL of solution, then the
(v/v)% concentration is
70 mL 2-propanol
100 mL rubbing alcohol x 100% = 70% (v/v)
12
Concentration Units
Using a Percent Concentration as a
Conversion Factor
Sample Problem 7.6
A saline solution used in intravenous drips contains
0.92% (w/v) NaCl in water. How many grams of NaCl are contained in 250 mL of this solution?
Step [1] Identify the known quantities and the desired quantity.
0.92% (w/v) NaCl solution
250 mL known quantities
? g NaCl desired quantity
13
Concentration Units
Using a Percent Concentration as a
Conversion Factor
Step [2] Write out the conversion factors.
100 mL solution
0.92 g NaCl or
0.92 g NaCl
100 mL solution
Choose this one to cancel mL.
Step [3] Solve the problem.
Answer
250 mL x
0.92 g NaCl
100 mL solution
= 2.3 g NaCl
Milliliters cancel.
14
Concentration Units
Parts Per Million
When a solution contains a very small concentration of solute, it is often expressed in parts per million .
Parts per million ppm = ppm = mass of solute (g) mass of solution (g) or x 10 6 volume of solute (mL) volume of solution (mL) x 10 6
15
Concentration Units
Molarity
Molarity is the number of moles of solute per liter of solution, abbreviated as M .
Molarity = M = moles of solute (mol) liter of solution (L)
16
Concentration Units
Molarity
HOW TO Calculate Molarity from a Given Number of
Grams of Solute
Example
Calculate the molarity of a solution made from 20.0 g of NaOH in 250 mL of solution.
Step [1]
Identify the known quantities and the desired quantity.
20.0 g NaOH
250 mL solution known quantities
? M (mol/L) desired quantity
17
Concentration Units
Molarity
HOW TO Calculate Molarity from a Given Number of
Grams of Solute
Step [2]
Convert the number of grams of solute to the number of moles. Convert the volume to liters if necessary.
• Use the molar mass to convert grams of NaOH to moles of NaOH (molar mass 40.0 g/mol).
20.0 g NaOH x
1 mol
40.00 g NaOH
= 0.500 mol NaOH
Grams cancel.
18
Concentration Units
Molarity
HOW TO Calculate Molarity from a Given Number of
Grams of Solute
• Convert milliliters of solution to liters of solution using a mL –L conversion factor.
250 mL solution x
1 L
1000 mL
= 0.25 L solution
Milliliters cancel.
19
Concentration Units
Molarity
HOW TO Calculate Molarity from a Given Number of
Grams of Solute
Step [3]
Divide the number of moles of solute by the number of liters of solution to obtain the molarity.
M = moles of solute (mol)
V (L)
=
0.500 mol NaOH
0.25 L solution
= 2.0 M
Answer
20
Dilution
Dilution is the addition of solvent to decrease the concentration of solute. The solution volume changes, but the amount of solute is constant. moles of solute (mol) = molarity (M) x volume (V)
M
1
V
1 initial values
= M
2
V
2 final values
21
Dilution
Sample Problem 7.13
How many milliliters of a 4.0% (w/v) solution must be used to prepare 250 mL of a 0.080% (w/v) solution?
Step [1]
Identify the known quantities and the desired quantity.
C
1
V
1
= C
2
V
2 initial values final values
C
1
= 4.0% (w/v) C
2
V
2
= 0.080% (w/v)
= 250 mL known quantities
? V(L) desired quantity
22
Dilution
Step [2]
Write the equation and rearrange it to isolate the desired quantity, V
1
, on one side.
C
1
V
1
= C
2
V
2
Solve for V
1 by dividing both sides by C
1
.
V
1
= C
2
V
2
C
1
Step [3] Solve the problem.
V
1
= (0.080%)(250 mL)
4.0%
= 5.0 mL dopamine solution
Answer
23
Osmosis
• The membrane that surrounds living cells is a semipermeable membrane .
• Semipermeable membranes allow water and small molecules to pass across, but ions and large molecules cannot.
• Osmosis is the passage of water across a semipermeable membrane from a solution of low solute concentration to a solution of higher solute concentration.
• Osmotic pressure is the pressure that prevents the flow of additional solvent into a solution on one side of a semipermeable membrane.
24
Osmosis and Biological Membranes
• Isotonic solutions .
• Two solutions with the same solute concentration/osmotic pressure are said to be isotonic.
• A 0.92% m/v sodium chloride solution is called a physiologic saline solution. It is isotonic with blood, i.e. has the same salt concentration as blood.
• Hypotonic solutions.
• A solution that contains a lower solute concentration compared to another is said to be hypotonic.
• Causes hemolysis ( bursting of red blood cells)
• Hypertonic solutions.
• Hypertonic solutions contain a higher solute concentration compared to another.
• Causes plasmolysis/crenation (shrinking of the red blood cells)
isotonic solution hypotonic solution
Hemolysis
(burst) hypertonic solution
Crenation
(shrink)
26