The Study - Tropical Medicine
advertisement
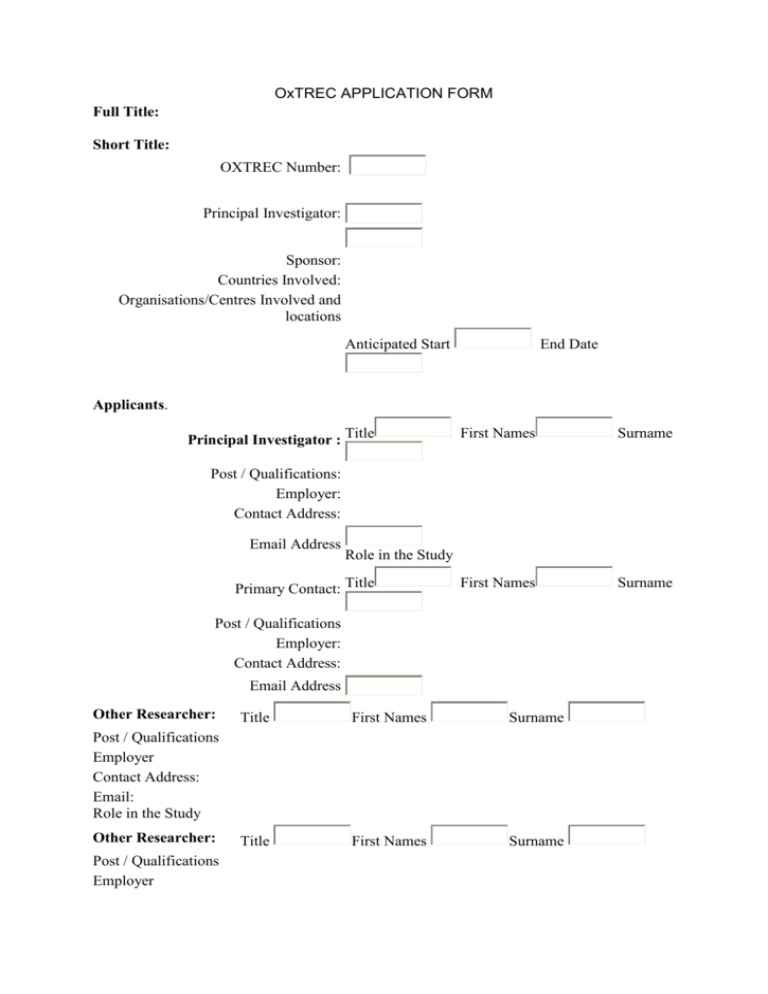
OxTREC APPLICATION FORM Full Title: Short Title: OXTREC Number: Principal Investigator: Sponsor: Countries Involved: Organisations/Centres Involved and locations Anticipated Start End Date Applicants. Principal Investigator : Title First Names Surname First Names Surname Post / Qualifications: Employer: Contact Address: Email Address Role in the Study Primary Contact: Title Post / Qualifications Employer: Contact Address: Email Address Other Researcher: Title First Names Surname Title First Names Surname Post / Qualifications Employer Contact Address: Email: Role in the Study Other Researcher: Post / Qualifications Employer Contact Address: Email: Role in the Study The Study 1. Brief Scientific Background and Rationale (max 500 words) 1.1. What is the primary objective of the study? 1.2. What is the primary endpoint or outcome measure? 1.3. What are the secondary objectives? 1.4. What are the secondary endpoints or outcome measures? Study design and methodology 2. Please summarise the Design and Methodology of the planned research (max 500 words. Please define abbreviations where necessary.) 3.1 Is this a clinical trial? For definition see: http://www.who.int/topics/clinical_trials/en/ 3.2 What is your intervention? eg. use of a new medical product or device, a surgical procedure, a therapy or a test. 3.3 Is a medical product being used outside its local terms of licence? 3.4 Does your trial need a Data and Safety Monitoring Board (DSMB)? 4. Please list all other procedures eg. blood and other samples taken, tests performed, questionnaires, etc. Procedure (For blood & CSF samples state volume & if venous, arterial, fingerprick etc) Number per person Routine Research Timing (eg. daily, weekly, over 3 months) Time taken per procedure Who will undertake it. 5. Give total volume of blood taken and over what time period: 6. Will participants’ genetic material be analysed? If so separate consent for this must be requested in the consent form. Recruitment and informed consent Screening process 7. What are the inclusion criteria of the study? 8. What are the exclusion criteria of the study? 9. Will subjects be recruited from any of the following groups? Pregnant Women: Children under 18: People with learning difficulties: Unconscious or severely ill: Other vulnerable groups, if yes please specify; 10. Identify any racial, ethnic, or gender group(s) which will be specifically excluded from participation in this research study and justify such exclusion. 11. How will the potential participants in the study be identified and approached? 12. How many participants will be recruited? Give the total sample size. 13. Describe groups and numbers per group. 14. How was the number of participants decided? 15. Has statistical advice been sought? Please specify and give details of trial statistical methods 16. How long will you allow potential participants to decide whether or not to take part? 17. Please give details of the Consent Process including details of who will take consent and how it will be done, with details of any information provided (a written information sheet, videos, or interactive material) Please submit copies. 18. Are there any issues that might affect consent? (for example ongoing trials in the same population or participants are very unwell). How would this be addressed? 19. Will there be advertising for recruitment eg. posters, flyers, e-mails? If so please provide copies. Risks and Benefits 20. What are the potential adverse effects, pain, distress, inconvenience, risks, or hazard for participants from the research procedures or changes to life style for participants? These may include tests, procedures, treatments and questionnaires. 21. Are there any potential benefits to participants? 22. Are there any risks or benefits to the community? Ethical issues 23. Summarise the main ethical issues from the participants and researchers point of view and say how you propose to address them. Confidentiality, Data Storage and Sample Storage 24. What measures will you take to ensure confidentiality of personal data, collection, storage access to and disposal of data? 25. Who will have control of and be the custodian for the data generated by the study? 26. Who will have access to participants’ or potential participants’ health records or other personal information? 27. For how long will data be stored at the end of the project and where? 28. What are the arrangements for storage and disposal of the biomedical samples (if applicable)? Payments and Incentives 29. Will participants receive reimbursement of expenses? If yes, state how much. Please convert to pounds or US dollars and relate to average daily wage if appropriate. 30. Will individual participants receive any payment or gifts for taking part in the research? If yes state how much and how this has been decided upon. 31. Will the cost of routine care be reimbursed? Management of the research 32. Has the proposed study had an independent peer review? If yes please attach copy of the review. If there were any comments, please include your response. 33. Give details of the local ethics committee to whom you have applied. If there is no appropriate local committee please provide an explanation. 34. Please give details of the competent authority (if any) eg regulatory agencies that will approve the study. 35. Will you inform any other health professional responsible for the participants’ care that they are taking part in the study? If yes please explain how, if no please justify. 36. What demand will this research place on local health services? How will the design of the project take these demands into account? 37. If the research sponsor is other than the University of Oxford, have they agreed to provide indemnity for the study? If not, please specify the alternative arrangements. 38. How will this research be funded? Please give details of the funding organisation, the amount secured, or the type of support to be provided and the duration of the grant or contract. 39. Will individual researchers receive any personal payment or other benefits for undertaking this research? If so, please provide full details. Are there any conflicts of interest? 40. Please indicate what training on research skills those working on this study have received, or will they receive. eg. GCP training, online training in ethics/ human subject protection etc. Publication and dissemination 41. How do you intend to report and disseminate the results of the study? 42. Will you inform participants of the results? 43. Additional Information for the Committee: Further requirement 44. Is the study funded by the US National Institutes of Health (NIH) or any other US federal funding agency? If so please complete the form for NIH Funded Studies (provide link to template)





