Vocabulary
advertisement

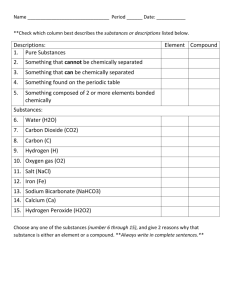
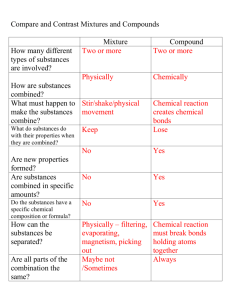
Vocabulary 1. __________________________: A quality of a substance that never changes and can be used to identify the substance. A characteristic property is a chemical or physical property that helps identify and classify substances. The characteristic properties of a substance are always the same whether the sample one is observing is large or small 2. ___________________________: the temperature at which a substance changes from a liquid to a gas The temperature at which a liquid boils and turns to vapor 3. ____________________: the temperature at which a substance changes from a solid to a liquid The temperature at which a given solid will melt 4. ___________________________: a change in a substance that does not change its identity; for example, a change of state. - A change from one state (solid or liquid or gas) to another without a change in chemical composition 5. ___________________________: a characteristic property of substance that indicates it ability to undergo a specific chemical change 6. ________________________: a change in which on or more substances combine or break apart to form new substances. Chemical change is any change that results in the formation of new chemical substances. At the molecular level, chemical change involves making or breaking of bonds between atoms. These changes are chemical: iron rusting (iron oxide forms 7. ____________________: Two or more substances that are mixed together but not chemically combined A product of mixing. Any combination or blend of different elements, kinds, qualities, etc.: Chemistry, Physics. An aggregate of two or more substances that are not chemically united and that exist in no fixed proportion to each other Solution: a very well-mixed mixture 8. ________________: a substance made of only on kind of matter and having definite properties A pure substance is a sample of matter with both definite and constant composition with distinct chemical properties 9. ___________________: a substance that cannot be broken down into other substances by chemical or physical means. each of more than one hundred substances that cannot be chemically interconverted or broken down into simpler substances and are primary constituents of matter. Each element is distinguished by its atomic number, i.e., the number of protons in the nuclei of its atoms. 10. ___________________: a substance made of two or more elements chemically combined A thing that is composed of two or more separate elements; a mixture. A substance formed from two or more elements chemically united in fixed proportions.