Lab 15: Kinetic Factors
advertisement

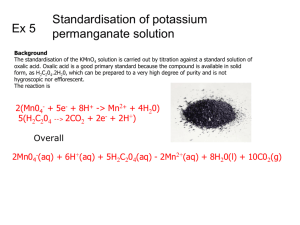
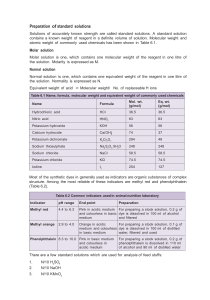
Kinetic Factors Experiment Name:________________________________________________________________________ Lab Partner:____________________________________________________________________ Problem Statements: What factors can be used to change the rate of a reaction? Are these factors predictable from the balanced equation? I. The Reaction: Permanganate Ion Reduced by Oxalic Acid In this experiment you will be studying the reaction between permanganate, MnO4-, and oxalic acid, C2O4H2, in acid solution. The permanganate is reduced to Mn+2 and the oxalic acid is oxidized to CO2. Write the balanced equation for the reaction: II. Watching the Reaction A. Using clean, dry, labeled 100 mL beakers, obtain 30 to 50 mL each of stock solutions 0.0014 M K2MnO4, 0.20 M C2O4H2, and 2.0 M H2SO4. Put about 50 mL of distilled water in a fourth beaker. Put a clean dropper in each beaker. You will be making up various mixtures of these four solutions by counting drops. Be careful that the droppers are not switched between beakers during any of the work. B. Take three clean, large test tubes and label them 1-3. You will be doing experiments three runs at a time and need to keep track of each run. Viewing them against a white background is advised. C. Make up the three solutions described below. Mix each by rapping on the test tube. Observe these solutions for about 10 minutes and record your observations. Run # KMnO4 H2 O H2SO4 C2O4H2 A1 15 15 15 0 A2 15 25 0 5 A3 15 10 15 5 D. Observations: E. Data Analysis: Offer explanations for your observations. Did all runs show a change? If not, why not? Did those that changed do so instantly? If not, why not? III. Data Collection: Effect of H+ Concentration A. In the experimental runs from here on, you will be timing how long it takes for all the MnO4-ions to react by watching the color fade. The recommended procedure is as follows: 1. Dump the current contents of your test tube into your waste beaker and rinse each a few times with about 1 mL of water. Shake out excess water. 2. Add the stock solutions to each tube as called for except for the C2O4H2 and mix. 3. Then quickly add the drops of C2O4H2 to each tube, mix, and record this as the starting time. Also record the time when the color has faded. 4. Starting Time Ending Time Total Run # KMnO4 H2 O H2SO4 C2O4H2 B1 15 20 5 5 B2 15 10 15 5 B3 15 0 25 5 B. Data Analysis: What effect does increasing the concentration of H+ ion have on the rate of the reaction? Does this seem reasonable? Explain your reasoning in terms of what the molecules and ions may be doing. III. Data Collection: Effect of C2O4H2 Concentration A. Make up the runs below using the procedure outlined above. Run # C1 C2 KMnO4 15 15 H2 O 10 5 H2SO4 15 15 C2O4H2 5 10 C3 15 0 15 15 Starting Time Ending Time Total A. Data Analysis: What effect does increasing the concentration of oxalic acid have on the rate of the reaction? Does this seem reasonable? Explain your reasoning in terms of what the molecules and ions may be doing. III. Data Collection: Effect of Copper Metal A. Before adding the C2O4H2 and recording the start time, add a length of copper metal. Make sure it is submerged in the liquid before starting the reaction. Run # D1 D2 KMnO4 15 15 H2 O 10 10 H2SO4 15 15 C2O4H2 5 5 Cu 0 2 in D3 15 10 15 5 4 in Starting Time Ending Time Total B. Data Analysis: What effect does increasing the presence and amount of copper metal have on the rate of the reaction? Does this seem reasonable? Explain your reasoning in terms of what the molecules and ions may be doing. III. Data Collection: Effect of Temperature A. Set up a water bath between 40-50 oC. Place the test tube with all the chemicals except oxalic acid in the water bath and allow to come up to temperature. Add the oxalic acid and watch the reaction. Perform the other experiment normally at room temperature. Run KMnO4 H2O H2SO4 C2O4H2 Temp Starting Time Ending Time Total # E1 15 10 15 5 20-30oC E2 15 10 15 5 40-50oC B. Data Analysis: What effect does increasing the temperature have on the rate of the reaction? Does this seem reasonable? Explain your reasoning in terms of what the molecules and ions may be doing. VII. Conclusions A. What could a chemist do to this redox reaction to speed it up or slow it down? Which factors are more effective? Which are less effective? Explain your reasoning. B. Could some or all the factors you found to affect the reaction rate have been predicted from knowing only the overall reaction? Explain your reasoning.