lno10234-sup-0003
advertisement

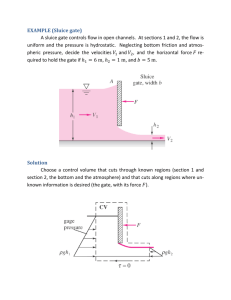
Supplemental information Microbial diversity and biogeochemistry in the marine cavity beneath the McMurdo Ice Shelf, Antarctica MIFlowCyt-Compliant Items (Lee et al., 2008) Requirement 1.1. Purpose 1.2. Keywords 1.4. Organization (name and address) 1.5. Primary contact (name and email address) 1.6. Date or time period of experiment 1.7. Conclusions 1.8. Quality control measures 2.1.2.1 Environmental Sample Description 2.1.1.2. Environmental Sample Location 2.2 Sample Characteristics Please Include Requested Information The aim of the present study is to quantify and characterize bacterial assemblages and autofluorescent phytoplankton in seawater samples from Ross Sea. Priscu Lab, Department of Land Resources and Environmental Sciences, 334 Leon Johnson Hall, Montana State University, Bozeman, 59717, MT, USA. Pamela Santibáñez (pamela.santibanez@msu.montana.edu), PhD student, MSU. Data collection was on the 25th and 27th of March, 2014. Bacterioplankton cell densities were 1.2 x 108 cells L-1 and 1.1 x 108 cells L-1 in the AASW and mHSSW water layers, respectively. Small (<2 mm) phytoplankton were more abundant in the surface water layer than the deep layer (1.49 x 10 6 cells L-1 and 1.00 x 104 cells L-1, respectively), while the densities of larger phytoplankton and total phytoplankton were 33% higher at depth. The flow cytometer (PhytoCyt from Turner Design) calibration was periodically inspected following the manufacturer instructions during the entire work. Milli-Q as blank control, negative control and background control were also performed. All samples were pre-filtered through 30μm mesh (sterile BD Falcon 12 x 75 mm Tube with Cell Strainer Cap) to eliminate large particles. The threshold levels were used to eliminate the inherent equipment noise floor. To detect heterotrophic bacterial events, threshold levels were set on green fluorescence channel (FL1-H) at 750 and on the forward-scatter channel at 250. To detect phytoplankton events, threshold levels were set on red fluorescence channel (FL4-H) at 625 and on Forward-scatter-H (FCS-H) channel at 250. Standard validation beads (Spherotech 8- and 6-Peaks Validation Beads) were run daily and prior to running samples to validate system fluidics performance and the instrument’s level of sensitivity in detecting events. Milli-Q water was used to wash the SIP and 3 backflushes were performed between samples to prevent carry-over. All control preparations (Milli-Q blanks, background and negative controls) were measured at the same instrument settings than samples. Background controls correspond to 0.2 μm filtered stained sample to recognize the background noise inherent to samples. Negative controls are unstained sample to detect autofluorescent events. Gating strategy was use to excluded the detected background noise and negative events. In addition, serial dilution curves were carried out to verify a linear correlation of both samples. Discrete water samples were collected through a borehole in the ice shelf on December 18, 2012 with a10 L General Oceanics Niskin bottle (AASW [30 m] sample) and a 5 L Go-Flo bottle (mHSSW [850 m] sample). The water was decanted immediately through acid leached silicone tubing into combusted glass bottles. Samples were preserved with 5% (v/v) sodium borate buffered formalin. 77.8902 S, 167.0083 E Samples were expected to contain prokaryotic and eukaryotic cells; the study focused on bacterioplankton and phytoplankton. The seawater was expected to contain low concentrations of particulate matter. 2.3. Sample Treatment(s) Description 2.4. Fluorescence reagent description 3.1. Instrument manufacturer 3.2. Instrument model 3.3. Instrument configuration and settings 4.2. Compensation description 4.3. Data transformation details 4.4.1. Gate description For the autofluorescent phytoplankton measurements, the formalin fixed samples were untreated. For total bacterial measurements, 1000 μL of each fixed sample was stained with SYBR® Green I (SGI) x1 final concentration, vortexed and incubated for 20 min in a sterile BD Falcon tube at ~22 °C (room temperature) in dark conditions (Marie, 1999). All samples were filtered prior to be stained through 30μm mesh (sterile BD Falcon 12 x 75 mm Tube with 30 μm Cell Strainer Cap) to eliminate large particles before flow cytometric analysis and prevent instrument clogging. Flow rate was 50μl min-1 and core size 12 μm. Background controls were filtered by Millex ®-GV4 filter units (4mm filter units, 0.1cm2 filtration area from Millipore) SYBR® Green I nucleic acid stain (Molecular Probes) was used to stain the seawater samples and detect bacterial cells. SYBR-green-I, a monomeric unsymmetrical cyanine dye and permeable to live cells; it has been used to quantify microorganisms in aquatic systems (Marie 1999; Gasol & Giorgio 2000). It has a fluorescence quantum yield of 0.80 for DNA and 0.40 for RNA, and it is maximally excited at 494 - 497 nm and has an emission maximum at 521 nm (Molecular Probes, Lebaron et al. 1998). It presents a fluorescence enhancement upon binding to DNA >1500 folds. In order to prepare different concentrations of stain solution, the stain was diluted in 0.2µm filtered 1x TBE (5.4gr Tris, 2.75gr Boric acid, 0.5M EDTA, pH 8.0) and prepared in a laminar flow hood. The optical detector green fluorescence (FL1; emission at 530±15 nm, excitation at 488 nm) was used to measure stained bacterial DNA. PhytoCyt Flow Cytometer was developed in partnership between Turner Designs and Accuri Cytometers (BD biosciences) http://www.bdbiosciences.com/instruments/accuri/index.jsp PhytoCyt (BD AccuriTM C6) Serial number: 3176 Technical specifications available at: http://www.bdbiosciences.com/documents/Accuri_C6_TechSpecs.pdf http://www.bdbiosciences.com/instruments/accuri/features/index.jsp The fluidics system uses peristaltic pumps to provide a non-pressurized, zero pulsation system. The flow cytometer electronics system provides 7 decades from 0 to 16,777,215 relative units of dynamic range eliminating the need to adjust detector voltage and gain settings. The flow cell is a 200 micron ID fused silica capillary. The fluorescence sensitivity is <750 MESF FITC and its precision is ≤3% CV for CEN. The instrument is equipped with two excitation lasers, four fluorescent and two scatter detectors: 488 nm excitation 20mW, solid state laser (Blue laser) Green fluorescence (FL1): Em. 530+/-15nm Yellow/Orange fluorescence (FL2): Em. 585+/-20nm Red fluorescence-blue laser dependent (FL3): Em. >670nm 640nm excitation; 30mW diode laser (Red laser) Red fluorescence-red laser dependent (FL4): 675+/-12.5 nm Scatter detections: Forward (0°, +/-15°) Side (90°, +/-15°) No compensation was performed CFlow Software has been used for the visualization, gating and data analysis. The gating strategy for bacterial events involved: GATE 1(DNA Stained Cells): Gate 1 is a polygon defined on a density-plot of Forwardscatter-A vs. SGI-H (FL1-H). Gate 1 has to be defined first to remove non-bacterial events (Figure P1). Gate 1 is set using a background control (0.2 μm filtered stained sample) and a negative control (unstained samples). Both controls are used to detect background noise, negative events, autofluorescent particles and cellular debris inherent to the sample. The gate 1 excludes those inherent no-target events and includes DNA stained events (SGI positive events). GATE 2 (Total Bacterial Cells): Gate 2 is a polygon defined on the same Forwardscatter-A vs. SGI-H density-plot than gate 1. Gate 2 is set using a stained sample. A SGI stained sample is run and all DNA stained events should fall in the gate 1. Then, the bacterial events appear as a distinguish cluster, and gate 2 is draw to identify the bacterial cluster. The total bacterial events are recorded in gate 2. Gate 2 should be created on the first 3000 detected events to discriminate bacterial cells from cellular debris and eukaryotic cells. To display the first 3000 events helps to distinguish the bacterial cluster and to draw gate 2 (gate 2; Figure P2). GATE 3 (Negative and positive SGI signal): Gate 3 is a vertical marker defined on the count vs. SGI-H histogram-plot (Figure P3). The vertical marker is set using negative controls (unstained sample) and SGI stained samples. The background control plus the negative control were used to exclude “background noise” in gate 1, but also to establish the vertical marker (gate 3). The negative control is unstained sample, the SGI signal should shift when the stain is applied. The negative control shows a negative SGI signal ((-) SGI) and the stained sample shows the positive SGI signal ((+) SGI). This vertical marker will be used to create gate 4, but it is also another visual control for (-) and (+) SGI signals. This vertical marker does not allow bacterial cluster discrimination by itself because there is small percentage of events that fall on (+) SGI, however it will help to create gate 7 and 8. GATES 4, 5 and 6 (Pico-phytoplankton discrimination from bacterial gate 2): Gates 4, 5 and 6 are quadrant markers in dot-plots. Gate 4 is defined on the forward-scatter-A vs. yellow/orange fluorescence (FL2-H; Em. 585+/-20 nm, Ex. 488 nm, Phycoerythrin); gate 5 is defined on the forward-scatter-A vs. red fluorescence (FL3; Em. > 670 LP nm, Ex. 488 nm; Chlorophyll-a ); and gate 6 is defined on the Forward-scatter-A vs. red fluorescence (FL4-H; Em: 675+/-12.5 nm, Ex. 640 nm; Phycocyanins). These quadrantgates show positive phytoplankton pigments in the bacterial cluster (gate 2) discriminating SGI stained pico-phytoplankton from the heterotrophic bacterial cluster, our goal (gate 2). The positive events of Phycoerythrin, Chlorophyll-a and Phycocyanins are excluded from gate 2, which leave only heterotrophic bacteria in gate 2 (Figure P4). GATES 7 and 8 (HNA and LNA bacteria): Gate 7 and 8 are horizontal markers defined on the count vs. SGI-H histogram-plot. Only the heterotrophic bacterial cluster (gate 2) was applied on the histogram-plot (picophycoplankton events were excluded). The horizontal markers are set using SGI stained sample after to setting gate 2. These markers discriminate between High Nucleic Acid (HNA) and low Nucleic Acid (LNA) bacteria. HNA and LNA are discriminated based on the SGI-H emission shift on the gated histogram-plot. The markers are created from the SGI-H shift to the left (gate 7; LNA) and to the right (gate 8; HNA; Figure P5). The same gates were applied for all the measurements. Gating boundaries for phytoplankton events: Chlorophyll gate: A background control (0.2 μm filtered unstained sample) is used to create chlorophyll gate and exclude background noise (Fig. P6). Then unstained sample is analyzed and phytoplankton cluster and subpopulations are visually gated (Fig. P6). The same gates were applied for all the measurements. 4.4.3. Gate boundaries Gating boundaries for heterotrophic bacterial events: Figure P1. Gate 1 is defined on a density plot of SGI-H fluorescence vs. forward-scatter-A (FSC-A) used to remove background noise, cellular debris, autofluorescent particles and negative events inherent to samples; a) Milli-Q sample as blank control; b) background control that corresponds to a 0.2 μm filtered stained sample; and c) negative control that corresponds to the sample without stain. Figure P2. Gate 2 defined on a density-plot of SGI-H vs. forward-scatter-A (FSC-A). Gate 2 is used to discriminate the bacterial cluster. (a) SG-I stained sample; the first 3000 detected events are displayed to easily draw gate 2. (b) All detected events are showed. Figure P3. Histogram plot of count vs. SGI-H fluorescence showing the vertical marker (vertical red line) that corresponds to gate 3, which is used to discriminate between (-) and (+) SGI signals and to define gate 7 and 8. a) MilliQ sample as blank control, b) background control (0.2 μm filtered SGI stained sample); c) negative control (unstained sample) and d) positive control (SGI stained sample). Figure P4. Cytograms of Phytoplankton pigments vs. forward-scatter-A (FSCA) from a SGI stained sample. Gates 4, 5 and 6 are used to remove picophytoplankton from the bacterial cluster (gate 2). (a) Positive Phycoerythrin gate ((+) Phy-E, gate 4), (b) Positive Chlorophyll-a gate ((+) Chl, gate 5) and (c) Positive Phycocyanin gate ((+) Phy-C, gate 6). The positive events from each pigment are excluded from the heterotrophic bacterial cluster (gate 2). Figure P5. Schematic representation of gates 7 (LNA) and 8 (HNA). As a first step the density-plot (A) of SGI-H vs. forward-scatter-A is used to gate the bacterial cluster (gate 2), pigments events from gate 4, 5 and 6 are excluded from this gate. Then, gate 2 is applied to the count vs. SGI-H histogram-plot (B) showing only the bacterial cluster. In (C), histogram-plot (B) is zoomed to visualize LNA and HNA clusters and to draw the horizontal markers that correspond to LNA (gate 4) and HNA (gate 5). (D) and (E) show LNA and HNA clusters in the SGI-H vs. Forward-scatter-A density-plot discriminated by the horizontal markers from plot (C). Gating boundaries for phytoplankton: . Figure P6. Density plots of chlorophyll fluorescence (FL3-H) vs. forward-scatter-A (FSC-A) on unstained sample. (a) (+) Chl gate is defined on 0.2 μm filtered unstained sample, which corresponds to background control. (b) Sample showing autofluorescent events, (c) phytoplankton cluster in (b) is zoomed to visualize different (+) Chl populations. Then populations are visually separated by gates in (d).