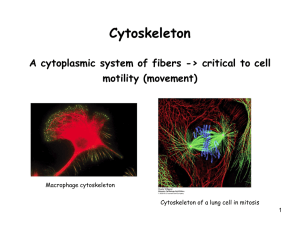
Motor proteins that transverse the microtubular cytoskeleton
advertisement

Gerald Karp Cell and Molecular Biology Fifth Edition Chapter 9: The Cytoskeleton and Cell Motility Copyright © 2005 by John Wiley & Sons, Inc. The study of the cytoskeleton Molecular motors Microtubules Intermediate filaments Actin filament Muscle contraction Non-muscle motility Skeleton: movement and support Cytoskeleton: movement and support Highly dynamic structure: m-RNA fixed on the cytoskeleton to process the transcription 9.1 Overview of the major function of the cytoskeleton Structural support that can determine the shape of the cell Positioning the organelles (polarity) Delivery the materials or organelles (mRNA) to the specific part of a cell Vesicles movement Cell locomotion Cell’s division machinery 9.2 The study of the cytoskeleton The use of fluorescence microscopy The use of video microscopy and laser beams for in vitro motility assays The use of cells with altered gene expression (a) The use of knockout animals (b) The use of cells over-express a dominant negative mutant protein 9.2 The study of the cytoskeleton The use of fluorescence microscopy The use of video microscopy and laser beams for in vitro motility assays The use of cells with altered gene expression (a) The use of knockout animals (b) The use of cells overexpress a dominant negative mutant protein Dominant negative mutant Cells produce large amounts of a nonfunctional protein. Cells are transfected to take up the altered DNA and incorporating it into their chromosomes. Once the cells have been genetically modified, the mutant protein either competes with the normal protein or interferes in some other way with its function, causing the cell to exhibit the mutant phenotype. A pigment cell from the xenopus Which treated with a hormone that induces dispersion of the pigment granules-lightening the skin A pigment cell that is overexpressing a gene for a mutant motor protein (kinesin II). The failure of the pigment granules to disperse in the cell indicating the Kinesin II as the motor protein responsible for the outward movement of the granules. (c). The use of small, double-stranded RNA molecules (siRNA) that complementary to the mRNA that encodes the particular protein being investigated. (Fig. 11.38) This RNA interference has become a common strategy in recent years to investigate the effect of a missing protein 9.3 Microtubules Structure and composion Hollow, tubular structure Mitotic spindle, flagella, cilia 25 nm (outer diameter) 13 protofilaments Functions pf microtubules As structural supports and organizers As agents of intracellular motility: axonal transport Functions pf microtubules As structural supports and organizers As agents of intracellular motility: axonal transport Motor proteins that transverse the microtubular cytoskeleton It has been known for several decades that microtubules are primarily structures that serve as track for a large number of motor proteins that generate the forces required to move objects within a cell. Convert chemical energy (ATP) to mechanical energy Motor protiens can be grouped into kinesins, dyneins (move along microtubules), and myosins (move along microfilaments) As the protein moves along, it undergoes a series of conformational changes that constitute a mechanical cycle coupled to the steps of a chemical cycle. The steps of chemical cycle include 1. binding of an ATP molecule to the motor 2. the hydrolysis of the ATP 3. the release of the proteins (ADP, Pi) from the motor 4. the binding of a new molecule of ATP 5. The binding and hydrolysis of a single ATP is used to drive a power stroke that moves the motor a precise number of nanometers along its track. A body of evidence suggests at least two roles for cytoplasmic dynein 1. a force-generating agent in the positioning of the spindle and movement of chromosomes during mitosis 2. a minus end-directed microtubular motor for the positioning of the Golgi complex and the movement of organelles, vesicles, and particles through the cytoplasm Microtubule-organizing centers (MOTCs) microtubule assembly in vitro: a. slow phase of nucleation b. elongation In vivo, nucleation of microtubules takes place rapidly inside the cell--microtubule-organizing centers The best-studied MOTC is the centrosome Centrosomes (in animals) 2 centrioles + pericentriolar materials (PCM) PCM: nucleation occurs Is the major site of microtubule initiation in animal cells and remain the cell’s microtubular network. In unpolarized cell, the centrosome is situated near the center of the cell. In polarized cell, microtubules are anchored by their – ends near the apical surface as + ends extend toward the cell’s basal surface. Basal bodies and other MOTCs 1. centrosomes are not the only MOTCs in cells 2. microtubules in a cilium or flagellum are generated in the structure called a basal body (identical in structure to centrioles) 3. basal bodies and centrioles can give rise to one another (centriole → basal body → flagellum during spermiogenesis, or sperm basal body → a centriole during the 1st mitotic division of the fertilized egg). 4. Plant cells only have MOTCs. Microtubule nucleation MOTCs γtubulin, 0.005 % of total cell protein The dynamic properties of microtubules Mitotic spindle fiber: liable (sensitive to disassembly) Mature neurons: less liable Cilia, flagella: highly stable Noncovalent association of dimeric building blocks Disassembly the microtubules Cold temp, hydrostatic pressure, elevated Ca 2+ conc., colchicine, vinblastine, vincristine, nocodazole Taxol: bind to polymer, inhibit disassembly, preventing from the assembling new microtubular structure Death of cancer cells due to the drugs effect on the assembly of spindle fiber in the cell division Recent research has revealed that normal cells arrest division until drugs eliminated. However, the cancer cells lack this mitotic check point and attempt to complete their division even in the absence of a functioning mitotic spindle → cell death The study of microtubule dynamics in vitro 1972, R. Weisenberg of Temple Univ. Crude brain homogenates at 37oC Adding Mg 2+, GTP, EGTA (bind to Ca2+, an inhibitor of polymerization) Found the microtubules could be disassembled and reassembled over and over by lowering and raising the temperature of medium The study of microtubule dynamics in vivo By microinjecting fluorescently labeled tubulin into a cultured cell Cilia and flagella: structure and function (only limit to the eukaryotes) How does a cell organize and maintain a construction site at the outer tip of an axoneme situated um from the cell body ? Intraflagellar transport (IFT) for assembling and maintaining the flagella 1. the protein responsible for conversion of the chemical energy of ATP into the mechanical energy of ciliary locomotion was isolated by Ian Gibbons of Harvard in 1960s 9.4 Intermediate filaments Antiparallel associate each other Highly dynamics in vivo Disappear before cell division, reappear in the daughter cell Assembly and disassembly related to phosphorylation and dephosphorylation (phosphorylation of vimentin cause disassembly) Functions of IFs Found around the nucleus Terminated in the cytoplasmic plaques of desmosomes and hemidesmosomes In nerve cells, called neurofilaments Japanese quail bearing mutation of neurofilaments possess axons that are markedly thinner than normal Functions of IFs Mice carrying deletions in type 1 keratin are so sensitive to mechanical response, can cause severe blistering of the skin or tongue An inherited human disease “desmin-related myopathy” patients suggested from skeletal weakness, cardiac arrhythmias and congestive heart failure 9.5 Microfilaments 7-8 nm, phalloidin or actin-Abfluorescent Migration, contraction, motility Wound-healing, axon growth, WBC migration, phygocytosis, cytokinesis G-actin → F-actin filament in the presence of ATP Highly ordered form (brush boarder) Ill-defined network Tightly anchored bundle (focal adhesion) Highly conserved Microfilament assembly and disassembly In vitro: ATP-actin monomers → growing end Barbed (+) end is the fast-growing end (high affinity to ATP-actin) Pointed (-) end is the slow-growing end (low affinity to ATP-actin) Cells maintain a dynamic equilibrium between the monomeric and polymeric forms of actin Cytochalasin (from mold) binds to + end of the actin filament allowing depolymerization at the – end. Phalloidin (from a poisonous mushroom) binds to intact filament and prevents their turn over. Latrunculin (from a sponge) binds to free monomers Myosin: the molecular motor of actin filaments Myosin superfamily (40+), generally divided into two groups: conventional (type II) and non-conventional. Move toward the + end of an actin filament Exist in mammalian cells, plants, nonmuscle cells, protists, vertebrate cardiac and smooth muscle cells 9.6 Muscle contraction The sliding filament model of muscle contraction The composition and organization of thick and thin filaments The energetics of filament sliding Excitation-contraction coupling T-tubules and SR Ca2+ and SER Ca2+ in SR 10-2 M Stimulus → T-tubules → Ca2+ releasing channel on SR→ Ca2+ release to the cytoplasm (10-5M) →troponin C → tropomyosin release from actin filament → exposure myosine binding site → combine with actin filament → contraction 9.7 Nonmuscle motility Contractile proteins are present in less ordered, more liable, transient arrangements. They are restricted to a thin cortex just beneath the plasma membrane. Phygocytosis, cell division, cell movement etc.