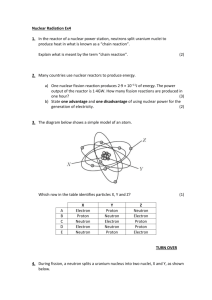
Radiation Detection and Counting Statistics
advertisement

Radiation Detection and Counting Statistics Please Read: Chapters 3 (all 3 parts), 8, and 26 in Doyle Types of Radiation • Charged Particle Radiation – Electrons • b particles – Heavy Charged Particles • a particles • Fission Products • Particle Accelerators Can be easily stopped/shielded! • Uncharged Radiation – Electromagnetic Radiation • g-rays • x-rays – Neutrons • Fission, Fusion reactions • Photoneutrons More difficult to shield against! Penetration Distances for Different Forms of Radiation a’s b’s g’s n’s Paper Plastic (few cm) Lead (few in) Concrete (few feet) Why is Radiation Detection Difficult? • • • • • Can’t see it Can’t smell it Can’t hear it Can’t feel it Can’t taste it • We take advantage of the fact that radiation produces ionized pairs to try to create an electrical signal Ideal Properties for Detection of Radioactivity Radiation Ideal Detector Properties a Very thin/no window or ability to put source inside detector Same as above, can be low or high density, gas, liquid, or solid High density, high atomic number materials Low atomic number materials, preferably hydrogenous b g neutrons How a Radiation Detector Works • The radiation we are interested in detecting all interact with materials by ionizing atoms • While it is difficult (sometime impossible) to directly detect radiation, it is relatively easy to detect (measure) the ionization of atoms in the detector material. – Measure the amount of charge created in a detector • electron-ion pairs, electron-hole pairs – Use ionization products to cause a secondary reaction • use free, energized electrons to produce light photons – Scintillators – We can measure or detect these interactions in many different ways to get a multitude of information General Detector Properties • Characteristics of an “ideal” radiation detector – High probability that radiation will interact with the detector material – Large amount of charge created in the interaction process • average energy required for creation of ionization pair (W) – Charge must be separated an collected by electrodes • Opposite charges attract, “recombination” must be avoided – Initial Generated charge in detector (Q) is very small (e.g., 10-13C) • Signal in detector must be amplified – Internal Amplification (multiplication in detector) – External Amplification (electronics) • Want to maximize V Q V C Types of Radiation Detectors • Gas Detectors – Ionization Chambers – Proportional Counters – Geiger-Mueller Tubes (Geiger Counters) • Scintillation Detectors – Inorganic Scintillators – Organic Scintillators • Semiconductor Detectors – Silicon – High Purity Germanium Gas Detectors • Most common form of radiation detector – Relatively simple construction • Suspended wire or electrode plates in a container • Can be made in very large volumes (m3) – Mainly used to detect b-particles and neutrons • Ease of use – Mainly used for counting purposes only • High value for W (20-40 eV / ion pair) • Can give you some energy information • Inert fill gases (Ar, Xe, He) • Low efficiency of detection – Can increase pressure to increase efficiency – g-rays are virtually invisible Ionization Chambers • Two electric plates surrounded by a metal case • Electric Field (E=V/D) is applied across electrodes • Electric Field is low – only original ion pairs created by radiation are collected – Signal is very small • Can get some energy information – Resolution is poor due to statistics, electronic noise, and microphonics Good for detecting heavy charged particles, betas Proportional Counters • Wire suspended in a tube – Can obtain much higher electric field – E a 1/r • Near wire, E is high • Electrons are energized to the point that they can ionize other atoms – Detector signal is much larger than ion chamber • Can still measure energy – Same resolution limits as ion chamber • Used to detect alphas, betas, and neutrons Examples of Proportional Counters Geiger Counters • Apply a very large voltage across the detector – Generates a significantly higher electric field than proportional counters – Multiplication near the anode wire occurs • Geiger Discharge • Quench Gas • Generated Signal is independent of the energy deposited in the detector • Primarily Beta detection • Most common form of detector No energy information! Only used to count / measure the amount of radiation. Signal is independent of type of radiation as well! Examples of Geiger Counters Geiger counters generally come in compact, hand carried instruments. They can be easily operated with battery power and are usually calibrated to give you radiation dose measurements in rad/hr or rem/hr. Scintillator Detectors • Voltage is not applied to these types of detectors • Radiation interactions result in the creation of light photons – Goal is to measure the amount of light created – Light created is proportion to radiation energy • To measure energy, need to convert light to electrical signal – Photomultiplier tube – Photodiode • Two general types – Organic – Inorganic } light electrons Organic Scintillators • Light is generated by fluorescence of molecules • Organic - low atomic numbers, relatively low density – Low detection efficiency for gamma-rays • Low light yield (1000 photons/MeV) - poor signal – Light response different for different types of radiation • Light is created quickly – Can be used in situations where speed (ns) is necessary • Can be used in both solid and liquid form – Liquid form for low energy, low activity beta monitoring, neutrino detection – Very large volumes (m3) Organic Scintillators Come in Many Forms Inorganic Scintillators • Generally, high atomic number and high density materials – NaI, CsI, BiGeO, Lithium glasses, ZnS • Light generated by electron transitions within the crystalline structure of the detector – Cannot be used in liquid form! • High light yield (~60,000 photons / MeV) – light yield in inorganics is slow (ms) • Commonly used for gamma-ray spectroscopy – W ~ 20 eV (resolution 5% for 1 MeV g-ray) – Neutron detection possible with some • Can be made in very large volumes (100s of cm3) Inorganic Scintillators Solid State (Semiconductor) Detectors • Radiation interactions yield electron-hole pairs – analogous to ion pairs in gas detectors • Very low W-value (1-5 eV) – High resolution gamma-ray spectroscopy • Energy resolution << 1% for 1 MeV gamma-rays • Some types must be cooled using cryogenics – Band structure is such that electrons can be excited at thermal temperatures • Variety of materials – Si, Ge, CdZnTe, HgI2, TlBr • Sizes < 100 cm3 [some even less than 1 cm3] – Efficiency issues for lower Z materials NaI Scintillator Ge Detector Ideal Detector for Detection of Radiation Radiation Ideal Detector a Thin Semiconductor Detectors Proportional Counters Organic Scintillators Geiger Counters Proportional Counters Inorganic Scintillators Thick Semiconductor Detectors Plastic Scintillators Proportional Counters (He, BF3) Lithium Glass Scintillators b g neutrons Excellent table on Page 61 shows numerous different technologies used in safeguards Counting Statistics Three Specific Models: 1. Binomial Distribution – generally applicable to all constant-p processes. Cumbersome for large samples 2. Poisson Distribution – simplification to the Binomial Distribution if the success probability “p” is small. 3. Gaussian (Normal) Distribution – a further simplification permitted if the expected mean number of successes is large The Binomial Distribution n = number of trials p = probability of success for each trial We can then predict the probability of counting exactly “x” successes: n! nx x Px p 1 p n x ! x! P(x) is the predicted “Probability Distribution Function” Example of the Binomial Distribution “Winners”: 3,4,5, or 6 P = 4/6 or 2/3 10 rolls of the die: n=10 Results of the Binomial Distribution p = 2/3 n =10 x pn 2 6 3 Some Properties of the Binomial Distribution n It is normalized: Px 1 x 0 Mean (average) value n x x Px x 0 x pn Standard Deviation “Predicted variance” n 2 2 x x Px x 0 “Standard Deviation” var iance is a “typical” value for x x For the Binomial Distribution: n! nx Px p x 1 p n x ! x! where n = number of trials and p = success probability Predicted Variance: n 2 2 x x Px x 0 Standard Deviation: np 1 p x 1 p x 1 p For our Previous Example p = 2/3 n = 10 x np 6 2 3 20 1 x 1 p 2.22 3 3 2 2 2.22 1.49 The Poisson Distribution Provided p << 1 x pn e pn Px x! pn x x e Px x x! x For the Poisson Distribution n Px 1 x 0 n Predicted Mean: x x Px x 0 x pn Predicted Variance: n 2 x x Px x 0 pn x Standard Deviation: 2 x Example of the Application of Poisson Statistics “Is your birthday today?” p 1 365 x x ex Px x! x pn 2.74 Example: what is the probability that 4 people out of 1000 have a birthday today? 2.74 e 2.74 P4 4 4 3 2 0.152 Discrete Poisson Distribution Gaussian (Normal) Distribution p << 1 Binomial Poisson x l arg e Poisson Px 1 2x x pn e 2 x x 2x Gaussian n Px 1 x 0 2 x x Example of Gaussian Statistics What is the predicted distribution in the number of people with birthdays today out of a group of 10,000? p 1 365 n 10000 Px 1 e 2 27.4 x 5.23 x 27.4 x 27.4 2 54.8 Distribution Gaussian Distribution The Universal Gaussian Curve to f(to) 0 0 0.674 0.500 1.00 0.683 1.64 0.900 1.96 0.950 2.58 0.990 Summary of Statistical Models For the Poisson and Gaussian Distributions: Predicted Variance: 2 x Standard Deviation: x CAUTION!! We may apply x only if x represents a counted number of radiation events Does not apply directly to: 1. Counting Rates 2. Sums or Differences of counts 3. Averages of independent counts 4. Any Derived Quantity The “Error Propagation Formula” Given: directly measured counts (or other independent variables) x, y, z, … for which the associated standard deviations are known to be x, y, z, … Derive: the standard deviation of any calculated quantity u(x, y, z, …) 2 u u 2u 2x 2y x y 2 Sums or Differences of Counts u=x+y or u = x - y 2 u u 2u 2x 2y x y 2 Recall: u 1 x u 1 y u 1 x u 1 y 2u 2x 2y u 2x 2y x y Example of Difference of Counts total = x = 2612 background = y = 1295 net = u = 1317 u 2612 1295 u 3907 62.5 Therefore, net counts = 1317 ± 62.5 Multiplication or Division by a Constant Example of Division by a Constant Calculation of a counting rate r x = 11,367 counts x t t = 300 s 11367 r 37.89 / s 300 s r x 11367 0.36 / s t 300 s rate r = 37.89 ± 0.36 s-1 Multiplication or Division of Counts Example of Division of Counts Source 1: Source 2: N1 = 36,102 (no BG) N2 = 21,977 (no BG) R = N1/N2 = 36102/21977 = 1.643 2 2 2 N1 N 2 R N1 N 2 2 2 N1 N 2 R N1 N 2 R 3 8.56 10 R R 5 7.32 10 R 2 R R R 0.014 R R = 1.643 ± 0.014 Average Value of Independent Counts Sum: = x1 + x2 + x3 + … + xN 2x1 2x 2 2x N x1 x 2 x N x Average: N N Single measurement: “Improvement Factor”: Nx N x x N x x 1 1 N N N For a single measurement based on a single count: Fractional error: x x 1 x x x x 100 1000 10,000 Fractional Error 10% 3.16% 1% Limits of Detection • In many cases within non-proliferation, you are required to measure sources that have a small signal with respect to background sources of radiation • Thus, we need to assess the minimum detectable amount of a source that can be reliably measured. • Let’s look at an example of testing the limits of detection Limits of Detection Two basic cases: No Real Activity Present Real Activity Present NS N T N B N s Counts from source N T Measured Counts N B Counts from background 2N s 2N T 2N B Limits of Detection – No Source Goal: Minimize the number of false positives (i.e., don’t want to holdup many containers that do not contain anything interesting) 2Ns 2N T 2N B 2N T 2N B 2Ns 22N B Ns 2 N B 2 N B if only fluctuatio ns from counting statistics Want to set critical counting level (LC) high enough such that the probability that a measurement Ns that exceeds Lc is acceptably small. Assuming Gaussian distribution, we are only concerned with positive deviations from the mean. If we were to accept a 5% false positive rate (1.645σ or 90% on distribution), then LC 1.645 NS 2.326 N B Limits of Detection – Source Present Goal: Minimize the number of false negatives (i.e., don’t want to let many containers that contain radioactive materials get through). Let ND be the minimum net value of NS that meets this criterion. We can then determine our lower critical set point. Let’s assume an acceptable 5% false negative rate. N D LC 1.645 N D But , N D N B , we can use the approximat ion N D 2 N B N D LC 2.326 N B N D 4.653 N B Assumes the width of the distribution of the source + background is approximately the same as that of the background only. In reality, these widths are not the same. Limits of Detection – Source Present ND 2N B N D ND 4.653 N B ND 2 N B 1 2 N B 1 4N B 4N B N D 2 N B 1.645 N D 4.653 N B 2.706 (Currie Equation ) ND a min imum det ectable activity fT f radiation yield per decay absolute det ection efficiency T measuremen t time Two Interpretations of Limits of Detectability • LC = lower limit that is set to ensure a 5% false-positive rate • ND = minimum number of counts needed from a source to ensure a false-negative rate no larger than 5%, when the system is operated with a critical level (or trigger point) LC that ensures a false positive rate no greater than 5% Neutron Detection Neutron Coincidence Counting Neutron Energy Classification Slow Neutron Detection Need exoenergetic (positive Q) reactions to provide energetic reaction products Useful Reactions in Slow Neutron Detection 10B (n, a) 7Li 6Li (n, a) 3H 3He (n, p) 3H (n, fission) The 10B(n,a) Reaction Q MeV 7 Li a 10 B n 7 * Li a 2.792 2.310 [10B (n, a) 7Li*] Conservation of energy: Eli + Ea = Q = 2.31 MeV Conservation of momentum: m Li v Li m a v a 2 m Li E Li 2 m a E a E Li 0.84 MeV E a 1.47 MeV Other Reactions Q MeV 6 Li n He a 3 He n3 H p X n, fission 3 4.78 0.765 ~ 200 Detectors Based on the Boron Reaction 1. The BF3 proportional tube 2. Boron-lined proportional tube 3. Boron-loaded scintillator The BF3 Tube • • • Typical BF3 pressure < 1 atm Typical HV: 2000-3000 V Usual 10B enrichment of 96% BF3 – Pulse Height Spectrum Boron-Lined Proportional Tube • Conventional proportional gas • Detection efficiency limited by boron thickness Boron-Lined Proportional Tube – Pulse Height Spectrum Fast Neutron Detection and Spectroscopy • Counters based on neutron moderation • Detectors based on fast neutron-based reactions • Detectors utilizing fast neutron scattering Moderated Neutron Detectors Moderating Sphere Moderating Sphere Neutron “Rem – Counter” Long Counter Long Counter Sensitivity Application of the 3He(n,p) reaction – the 3He Proportional Tube 3He Proportional Counter Detectors that Utilize Fast Neutron Scattering 1. Proton recoil scintillator High (10 – 50%) detection efficiency, complex response function, gamma rejection by pulse shape discrimination 2. Gas recoil proportional tube Low (.01 - .1%) detection efficiency, can be simpler response function, gamma rejection by amplitude 3. Proton recoil telescope Very low (~ .001%) detection efficiency, usable only in beam geometry, simple peak response function 4. Capture-gated spectrometer Modest (few %) detection efficiency, simple peak response function Proton Recoil Scintillators Recoil Proton Spectrum Distortions Recoil Proton Detector Efficiency Proton Recoil Telescope Proton Recoil Telescope Response Function Ep = Encos2 θ Capture-Gated Proton Recoil Neutron Spectrometer Capture-Gated Spectrometer: Timing Behavior Accept first pulse for analysis if followed by second pulse within gate period Capture-Gated Spectrometer: Response Function • Only events ending in capture deposit the full neutron energy • Energy resolution limited by nonlinearity of light output with energy (Two 0.5 MeV protons total yield less than one 1 MeV proton.) Neutron Coincidence Counting • Technique involving the simultaneous measurement of neutrons emitted from a fission source (in “coincidence” with each neutron) • Used to determine mass of plutonium in unknown samples – Most widely used non-destructive analysis technique for Pu assay, and can be applied to a variety of sample types (e.g., solids, pellets, powders, etc.) – Requires knowledge of isotopic ratios, which can be determined by other techniques – Also used in U assay Neutron Distribution from Pu Fission Neutron Coincidence Counting • Makes use of the fact that plutonium isotopes with even mass number (238, 240, 242) have a high neutron emission rate from spontaneous fission – Spontaneous fission neutrons are emitted at the same time (time correlated), unlike other neutrons (a,n), which are randomly distributed in time – Count rate of time correlated neutrons is then a complex function of Pu mass Fission Emission Rates for Pu isotopes Isotope Spontaneous Neutron Emission Rate (neutrons/sec-g) Pu-238 2.59 x 103 Pu-239 2.18 x 10-2 Pu-240 1.02 x 103 Pu-241 5 x 10-2 Pu-242 1.72 x 103 In reactor fuel, Pu-240 signal dominates over Pu-238 and Pu-242 due to abundance Neutron Coincidence Counting • In neutron coincidence counting, the primary quantity determined is the effective amount of Pu-240, which represents a weighted sum of the three even numbered isotopes m240eff a m238 m240 c m242 • Coefficients for contributions from Pu-238 and Pu-242 are determined by other means, such as knowledge of burnup of reactor fuel. Without additional information, calculation will have errors but will give a good estimate of Pu mass due to relative abundance of the three isotopes. Generally, a ≈2.52, c ≈ 1.68 Neutron Coincidence Counting • In order to determine the total amount of Pu, mPu, the isotopic mass fractions (R) must be known. These can be easily determined through massspectroscopy or gamma-ray spectroscopy, and is then used to calculate the quantity 240 Pu eff aR 238 R 240 cR 242 m Pu m 240eff 240 Pu eff NCC Technique • Utilize He-3 detectors, which can moderate and detect spontaneous fission neutrons • He-3 detectors usually embedded in neutron moderating material to further slow down neutrons – Increases detection efficiency • Most common measurement is the simple (2-neutron) coincidence rate, referred to as doubles – If other materials present in the material contribute to neutron signal, or impact neutron multiplication, other effects may become significant, producing errors – Generally carried out on relatively pure or well characterized materials, such as Pu-oxides, MOX fuel pins and assemblies NCC Counters NCC Sources of Uncertainty • Counting statistics (random) – Can be a significant issue since efficiency can be low • Calibration parameters and uncertainties associated with reference materials (systematic) • Correction for multiplication effects, detector dead time, other neutron emission (systematic) • Nuclear data NCC Parameters to Consider 1. 2. 3. 4. 5. 6. 7. 8. 9. Spontaneous fission rate Induced fission (a,n) reaction rate Energy spectrum of (a,n) neutrons Spatial variation of multiplication Spatial variation of detection efficiency Energy spectrum effects on efficiency Neutron capture in the sample Neutron die-away time in the detector Clearly, there can be more unknowns than can be determined in conventional NCC NCC Parameters • We want to determine 1,2,3 • 4 and 5 can be determined with proper use of modeling and simulation • 6 and 7 can be determined through proper calibration • 8 and 9 are usually unknown, but in general, are of minor consequence • Traditional NCC can end up indeterminate – only 2 equations, but three unknowns Neutron Multiplicity Measurements • In neutron multiplicity counting (NMC), one utilizes triple coincidence rates (in addition to single and double counting rates) to provide a third measurement such that all parameters can be determined • Thus, we are solving three equations with three unknowns – solution is self contained and complete • One significant advantage of NMC is that there is no need for careful calibration with Pu standards – Also, can measure samples where there may be significant uncertainties in composition Design of NMC • • • • • Maximize detection efficiency Minimize signal processing time Minimize detector die-away time to decrease accidental coincidences Minimize geometry effects to efficiency Minimize spectral effects on efficiency Advantages of NMC • Greater accuracy in Pu mass determination • Self-multiplication and (a,n) rates are directly determined • Calibration does not necessarily require representative standards • Measurement time on the order of a few thousand seconds, shorter than the 10,000s typical of NCC • Higher efficiency NMC systems can provide even shorter measurement times with improved accuracy Disadvantages of NMC • Cost • More floor space required • Some other techniques can provide shorter measurement times • Some biases can remain if there is a high degree of uncertainty in measured samples • Running out of He-3 Examples • In-Plant NMC measurement system Examples • 30-gallon drum measurement system Examples • High efficiency neutron counter