Lecture 9
advertisement
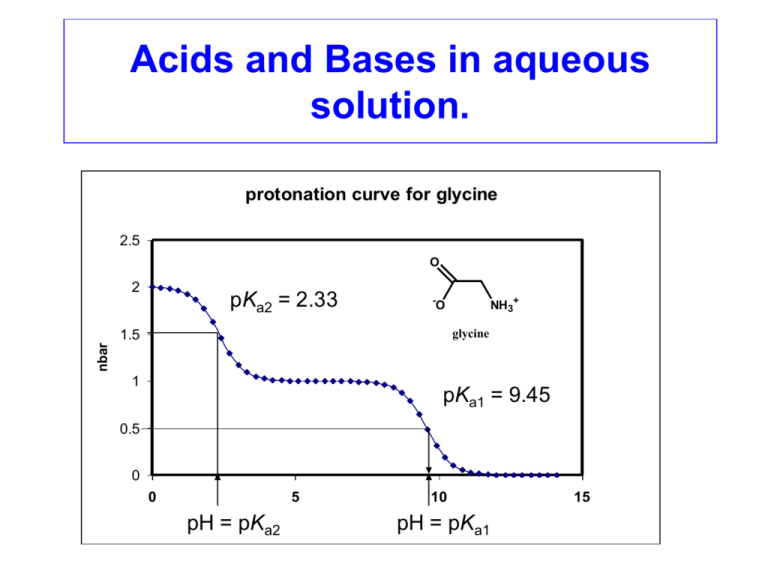
Acids and Bases in aqueous solution. protonation curve for glycine 2.5 O 2 pKa2 = 2.33 - NH3+ O 1.5 nbar glycine 1 pKa1 = 9.45 0.5 0 0 5 pH = pKa2 10 pH pH = pKa1 15 The properties of water: ~ 2.75 Å H-bonds Water is held together by H-bonding, which gives it its surprisingly high B.Pt. and M.Pt. for such a small molecule. Note that the heavier H2S molecule is a gas at room temperature. Water is also weakly ionized into H+ and OH- ions, as discussed next. The self-ionization of water: Water ionizes weakly to produce OH- and H+ ions, where Kw is the ionization constant of water, according to : H2O(l) H+ (aq) + OH-(aq) Kw = 10-14 This means that the product of the concentration (molarity) of [H+] and [OH-] must always be 10-14. Kw = 10-14 = [H+] [OH-] [1] Thus, for pairs of [H+] and [OH-] concentrations in solution we have: [H+] = 1.0 M, then [OH-] = 10-14/1.0 [H+] = 0.01 M, then [OH-] = 10-14/0.01 [H+] = 10-7 M, then [OH-] = 10-14/10-7 [H+] = 10-12 M, then [OH-] = 10-14/10-12 [H+] = 10-15 M, then [OH-] = 10-14/10-15 = 10-14 M = 10-12 M = 10-7 M = 10-2 M = 10 M pH and pOH The concentration of [H+] and [OH-] can be expressed as the pH and pOH: (p in pH or pOH means ‘potency’, and means that any constant ‘K’ or concentration with a ‘p’ in front of it is represented as pK = -log K , or pH = -log [H+]) pH = - log [H+] pOH = - log [OH-] We can show that: pKw = 14.0 = pH + pOH Thus, for any solution where the pH is known, pH + pOH = 14.0, or pOH = 14 – pH. [H+] pH pOH [OH-] 10 M 1M 0.01 M 10-5.7 M 10-7 M 10-9.3 M 10-12 M 10-14 M 10-15.09 M -1.0 0.0 2.0 5.7 7.0 9.3 12.0 14.0 15.09 15.0 14.0 12.0 8.3 7.0 4.7 2.0 0.0 -1.09 10-15 M 10-14 M 10-12 M 10-8.3 M 10-7.0 M 10-4.7 M 10-2 M 1M 12 M Acid dissociation constants (Ka): A weak Brønsted acid such as acetic acid (CH3COOH) will dissociate to give off protons in aqueous solution according to: acetic acid acetate ion CH3COOH (aq) CH3COO- (aq) + H+ (aq) conjugate acid proton conjugate base The extent of such dissociation is controlled by the acid dissociation constant, Ka. Ka = [ CH3COO- ] [ H+ ] [ CH3COOH ] The acid dissociation constant of acetic acid: The value of Ka for acetic acid is 10-4.84, so the pKa is 4.84. This gives us the expression: 10-4.84 = [ CH3COO- ] [ H+ ] [ CH3COOH ] [2] This expression can be used to solve problems relating to the ionization of acetic and other acids. For example, what is the pH of a 0.1 M solution of acetic acid? Equation 2 must be obeyed at all times, so we have: 10-4.84 = [CH3COO-][H+]/[0.1] = [H+]2/[0.1] so [H+] = (10-4.84 x 0.1)0.5 = 10-2.92 M, pH = 2.92. Notice that in solving the above problem, ionization of acetic acid produces equal concentrations of H+ and CH3COOions. One assumes that the amount of ionization is small, so that [CH3COOH] is not corrected for the small amount that it decreases, and we assume that [CH3COOH] is still ≈ 0.1 M. b) What is the pH of a 1 M solution of ammonia if the pKa of NH4+ is 9.2? Note: pKa of an acid plus pKb of its conjugate base = pKw pKa(NH4+) + pKb(NH3) = pKw = 14.0. pKb NH3 = 14.0 – 9.2 = 4.8, so Kb = 10-4.8. 10-4.8 = [NH4+] [OH-] / [NH3] so again we have: [OH-] = (10-4.8 x 1)0.5, = 10-2.4. If [OH-] = 10-2.4 M, then pOH = 2.4, and pH = 14-2.4 = 11.6 The species distribution diagrams of acids and bases: We can calculate the percentage of an acid (e.g. CH3COOH) or its conjugate base (CH3COO-) that is present as the acid or the conjugate base at any given pH value if the pKa is known (4.84). Thus, for acetic acid at pH 3.7 we have: 10-4.84 = [CH3COO-] [H+] / [CH3COOH] = [CH3COO-] [10-3.7] / [CH3COOH] [CH3COO-] / [CH3COOH] = 10-4.84 / 10-3.7 = 10-1.14 = 0.072 so percent of [CH3COO-] at pH 3.7 = 100% x (0.072/(1+0.072)) = 7.2 %. % of [CH3COOH] = 100 – 7.2 = 92.8 %. percent as species indicated. species distribution vs pH acetic acid 110 100 90 80 70 60 50 40 30 20 10 0 CH3COO- CH3COOH pH50 = pKa 2 3 4 5 pH 6 7 8 The relationship between pH50 and the pKa The pH at which the concentrations of CH3COOH and CH3COO- in solution are equal, i.e. both are 50%, is known as the pH50. On the previous slide it was indicated that the pKa of the acid equals pH50. This arises simply as follows: Ka 10-4.84 or pKa = 10-4.84 = = = [ H+ ] pH50 [ CH3COO- ] [ H+ ] [ CH3COOH ] [CH3COO-] = [CH3COOH] at pH50, so these cancel Thus, we saw that for CH3COOH the crossover point for [CH3COOH] and [CH3COO-] occurred at pH 4.84, which is the pKa. Similarly, on the next slide, we see that the crossover point for [NH4+] and [NH3] occurs at pH 9.22, which is the pKa of NH4+. species distribution vs pH of The species distribution diagram of ammonia/ammonium ion percent as species indicated. ammonia/ammonium ion: 110 100 90 80 70 60 50 40 30 20 10 0 NH4+ NH3 pH50 = pKa 5 7 9 pH 11 13 Multiprotic acids and bases: Many acids have more than one ionizable proton, and many bases have more than one proton acceptor site. A familiar example of this is triprotic phosphoric acid: H3PO4 H2PO4HPO42- H2PO4- + HPO42- + PO43+ H+ H+ H+ pKa3 pKa2 pKa1 = = = 2.15 7.20 12.38 It can be shown that for a monoprotic acid, when the concentration of the acid and its conjugate base are equal (both 50%), then pKa = pH. For polyprotic acids the average extent of protonation at any pH is given by the function nbar. For the phosphate ion: nbar = average number of protons bound per phosphate The relationship between nbar, pH, and the pKa values of multiprotic acids On a previous slide we showed that for a monoprotic acid such as CH3COOH, the pKa = pH50. At pH50 for a monoprotic acid, in fact, nbar = 0.5. This is the point at which half of the acetate ions have a proton on them, while half do not. For polyprotic acids, similar considerations apply. At nbar = 0.5 for phosphate, we have [HPO42-] = [PO43-], and the pH at nbar = 0.5 = pKa1. In fact the half-values of nbar give us the pKa values from the corresponding pH values: nbar pH = pKan 0.5 1.5 2.5 pH = pKa1 pH = pKa2 pH = pKa3 etc. Thus from a curve of nbar versus pH for phosphoric acid, we can estimate the pKa values from the pH values corresponding to nbar = 0.5, 1.5, and 2.5: H3PO4 protonation Plot of nbar versus pH forcurve phosphate: 3.5 H3PO4 3 pKa3 = 2.15 nbar 2.5 H2PO4- 2 pKa2 = 7.20 HPO42- 1.5 pKa1 = 12.38 1 PO43- 0.5 0 0 5 pH = pKa3 pH pH pH = pKa2 10 15 pH = pKa1 Species distribution diagram for phosphoric acid H3PO4 H2PO4- HPO42- pH PO43- protonation curve for glycine 2.5 2 O O O HO NH3+ - NH3+ O - O NH2 glycine 1.5 nbar pKa2 = 2.33 1 pKa1 = 9.45 0.5 0 0 5 pH = pKa2 10 pH pH = pK a1 pH 15 O- O- O O - O + NH+ -H OH NH+ - + O H O- + N - O O pKa2 = 6.13 -H+ HN - O - pKa1 = 9.46 O -H+ - O NH+ +HN O - O O - O O- O O H+ - O HO pKa3 = 2.69 (nitrilotriacetate) O N O- N - O O O EDTA O -H+ NTA O O O H+ pKa1 = 9.52 O H+ O - O O- O- N H O O -H + pKa2 = 2.52 - O O + O O O O- O O NH+ +HN O - O O- O (ethylenediamine tetraacetate) Lewis acids and bases: You will recall Brønsted acids and bases, where a Brønsted acid is a proton donor and a Brønsted base is a proton acceptor. A broader definition is that of Lewis Acids and Bases, where a Lewis Acid is an electron acceptor, and a Lewis Base is an electron donor. F-, a Lewis Base BF3, a Lewis Acid Gilbert Newton Lewis (1875-1946) Lewis Acid accepts electrons from the Lewis Base to form a complex.