Cell viabilty
advertisement
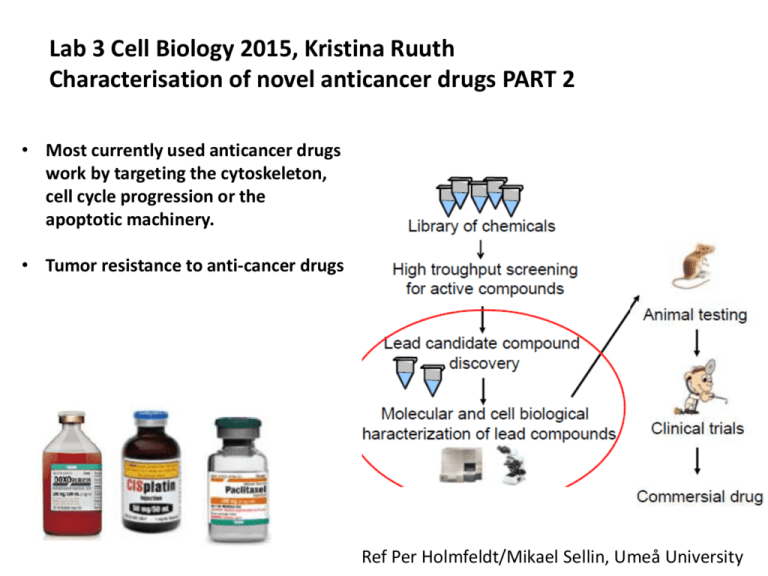
Lab 3 Cell Biology 2015, Kristina Ruuth Characterisation of novel anticancer drugs PART 2 • Most currently used anticancer drugs work by targeting the cytoskeleton, cell cycle progression or the apoptotic machinery. • Tumor resistance to anti-cancer drugs Ref Per Holmfeldt/Mikael Sellin, Umeå University Lab3: Each group will test 7 substanses from a chemical library plus 1 solvent control on Jurkat cells Cell viabilty: 1. Tryptan blue exclusion after 24 and 48 h. 2. Metabolic activity: Cell mediated reduction of resazurin after 24 h. Giemsa stain. FACS analysis: Cells double stained with immuno fluorescence technique for micro tubules and PI for cell cycle status. Dipak M et al Nature Comm 2013 Cell viabilty: Trypan blue exclusion: Counting of trypan blue stained cells in a burker chamber. Count all samples in triplicate at 2 time points , 24 h and 48h after start of treatment. Triplicate: Count viable cells in at least 3 “A-squares” calculate average . Repeat this twice for each treatment and the solvent control. Cell conc 0.5x106 cell/ml Diluted 1:2 will result in approx 25 cells/A-Square with large fluctuations. Hints: Dilute 4:5 , eg 200uL cells + 50 uL Trypanblue . Take out samples for counting and place the tubes on ice. Storage on ice for a couple of hours will not affect cell viability. Cell Viability Metabolic activity, Proliferation assay. Many assays: MTT, WST, 3H-thymidine, Brdu incorporation, Resazurin. Resazurin (Alamar Blue): Untreated cells + resazurin Medium + resazurin Analyse all samples, in triplicate wells. Seed 150 uL/well, according to template, 96-well plate, 0.5x106 cells/mL Incubate 24h, add 50 uL/well of resazurin (final conc in wells 55 uM) incubate 2-3h 37°. Read generated fluorescence in Tecan M200 multiplate reader . Excitation set to 530 and emission set to 590 nm. Resazurin stock solution 4.4 mM. The reader is booked for 1hr at 3 occasions on Tuesday 10/3: 12:00-13:00, 14:00-15:00, 16:00-17:00 530nm 590nm 3. Giemsa/May Grunewald stain. Consider results from the two viability assays “trypan blue exclusion” and “resazurin assay.” Which samples from the chemical library affected growth? Choose 4 samples together with the solvent control and spin out cells on slides and stain cells with May Grunewald/Giemsa according to instructions given in Lab 1. After staining look through the slides and search for a phenotype for that specific drug. Estimate ratio “phenotypic cells”/normal cells among 100-150 cells. Methods to distinguish out stages in the cell cycle: G0 from G1. Ki-67 is not expressed in G0 cells R G0/G1 S G2/M Has cells passed the G1 restriction point. Western blot of cell lysates with Rb-antibodies Rb pRb actin How to resolve different stages within G2/M G0/G1 S G2/M FACS analysis of micro-tubules Jurkat cells μ-tubules Control Drug Ref Dipak M et al Nature Comm 2013 Intact cells Ref: Per Holmfeldt, Umeå university Perforation of plasma membrane in presence of saponin and taxol; Unpolymerised tubulins will diffuse out of the cell After fixation incubate cells with anti-tubulin antibodies followed by incubation with FITC-labelled secondary antibodies. Staining of microtubules for FACS (copied from “-MG lab methods 2011-“) Solutions: MT-STAB PEM buffer, pH 6.9 0.1 % Saponin (stock 10%) 10 µg/ml RNase (stock 5mg/ml) 50 nM Taxol (stock 1,1mM) MT-FIX ~50 % PEM buffer, pH 6.9 ~50 % PFA in H2O (from 8% stock) 0.1 % Saponin (add 1/100 of a 10 % stock, which may be stored ~1 week) BLOCK 90 % PBSA / 10 % FCS / 0,1 % Saponin WASH PBSA / 0,1 % Saponin PI SOL. PBSA 10µg/ml Propidium Iodide 0,1% Triton X-100 (add 1/100 of a 10 % stock) 10µg/ml RNase 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Spin down 500.000 cells per FACS tubes for 2 min, 1100rpm (250xg). Aspirate the supernatant and resuspend the pelleted cells gently using the shaking table. Extraction of cells; add 400µl 37 °C MT-STAB and incubate at 37 °C for 4 minutes. Fixation of cells; add 400 µl 37°C MT-FIX and incubate at 37 °C for 12 minutes. Spin down the cells, 3 min, 1100rpm. Aspirate the supernatant and resuspend the cells by shaking. Add 1 ml BLOCK and incubate at 37 °C for 10 minutes. Spin down the cells, 5 min, 1100rpm. Aspirate the supernatant and resuspend the cells by shaking. Add 150 µl primary antibody (anti--tubulin T5168 B-5-1-2 dil 1:250 in BLOCK). NOTE! Include a tube with no addition of primary antibody to define the background. Incubate at 37 °C for 60 minutes while shaking. Add 1 ml WASH. Spin down the cells, 5 min, 1100rpm. Aspirate the supernatant and resuspend the cells by shaking. Add 100 µl secondary antibody (Rabbit-anti-mouse (FITC) DAKO F0313 dilute 1:20 in WASH). Incubate at 37 °C for 30 minutes while shaking. Add 1 ml WASH. Spin down the cells, 5 min, 1100rpm. Aspirate the supernatant and resuspend the cells by shaking. Add 500µl PI SOL and keep samples in refrigerator until analysis. micro-tubules är very britle Aspirate medium 500 μL cells Loosen pellet by gently shaking on shakerboard Extraction of “free” tubulin Add 400 μL MT-STAB 37° inc 37° 4 min. Fixation of cells Add 400 μL MT-FIX 37° inc 37° 12 min Centrifuge 1100 rpm 3 min Centrifuge 1100 rpm 2min Aspirate sup and shake gently Blocking of unspecific protein binding sites. Add 1ml BLOCK and inc 10min 37° Aspirate sup and shake gently Add 150 μL Mouse anti-α–tubulin Antibody and inc 60min 37° in shaking waterbath. Centrifuge 1100 rpm 5 min Aspirate sup and shake gently Add 100 μL Secondary ab: FITC Rabbit α mouse IgG, inc 30min 37° in shaking waterbath Add 1 mL WASH Centrifuge 1100 rpm 5 min Add 1 mL WASH Centrifuge 1100 rpm 5 min Aspirate sup and shake gently Add 0.5ml PI-soution. Store cells in fridge until FACS analysis Structural effects on microtubules After FACS analysis of cells stained for u-tubules Adjust cell cons to approx 0.5x106/mL and resuspend the cells. Spinn out 0.1x106 cells onto slides. Mount with “anti-fade” mounting medium and cover with coverslip. Let dry until next day an inspect the micro-tubule structure with a fluorescence microscope. Describe the structrual changes, count the phenotypic cells vs normal cells. Microscope slides with wells an alternative to cytospinning Adjust cellconc to approx 0.5x106/mL. Label wells on slide, 12 wells/slide and put it into ”humified chamber”. Pipet 25 μL/well of resuspended stained cells in PI-sol. Allow cells to sediment onto glass for 2h in the dark. Remove excess of solution with a small piece of 3MM, add a drop of “anti-fade” mounting medium to the well and cover with a round coverslip 11mm diameter. Safety issues: • PFA (paraformaldehyde) is skin and airway irritant and could cause allergic reactions! Wash exposed skin areas with water and soap. • May Grunewald staining solution is flammable and skin irritant. Rinse with ethanol followed by water in large amounts! • Giemsa is skin irritant. Rinse with ethanol followed by water in large amounts! • PI (propidium iodide) binds to DNA and should be handle with care. Rinse with ethanol followed by water in large amounts! • All drugs are cytotoxic drugs. Rinse with ethanol followed by washing of exposed areas with water and soap. Group wise presentation: Fully planned experiments Reagents and supplies and amount needed as well as a time schedule Drugs: 200x final conc. Jurkat cells: Amount of cells and RPMI1640 10% FCS. Reagents : MT-STAB, MT-FIX, PI-solution, trypan blue solution, resazurin 4.4 mM, saponin 10%, antibodies, buffers…………………………………………….. Other supplies Tubes Epp-tubes, multiwell plates, slides, cover slips ……………………………………………. Equipment: You do not need to include pipett tips , disposable pipets, May Grunewald and Giemsa staining solutions and mounting media. Carefully planned time schedule for Monday 10/3 Tuesday 11/3. Calculations how to obtain original cell conc when cells are diluted 200ul cells + 50 ul trypanblue. Format for seeding cells into 96-well plate for resazurin Time Slots for group discussions: Monday 10/3 group 8:00 15, 16, 17 8:30 12,13, 14 9:00 9, 10, 11 12:00 12:30 13:00 6, 7, 8 3, 4, 5 1, 2 C C C C C C S1 S3 S5 S7 S1 S3 S5 S7 S1 S3 S5 S7 S2 S4 S6 B S2 S4 S6 B S2 S4 S6 B C Solvent control S1 Cells treated with drug #1 S2 Cells treated with drug #2 and so on B culture medium without cells




