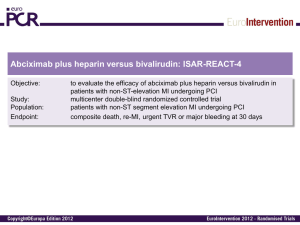
An additional analysis will be done for the stent thrombosis endpoint
advertisement

Statistical Analysis Plan ANGIOX® (BIVALIRUDIN) PROTOCOL NO. TMC-BIV-08-03 E.U.R.O.M.A.X EUROpean aMbulance Acs angioX trial CONFIDENTIAL Property of The Medicines Company May not be used, divulged, published or otherwise disclosed without the consent of The Medicines Company Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 2 of 23 Statistical Analysis Plan Approval Protocol No. TMC-BIV-08-03 EUROpean aMbulance Acs angioX trial (EUROMAX) Prepared By: _________________________________________________________________ Debra Bernstein, Ph.D. Date Senior Director, Biostatistics Reviewed and Approved by: ______________________________________________________________ Tristan Hu, Ph.D. Date Senior Director, Statistical Operations _________________________________________________________________ Efthymios Deliargyris, M.D. Date VP, Global Medical _________________________________________________________________ Diana Schuette, Ph.D. Date Senior Clinical Project Manager _________________________________________________________________ Judy Sromovsky Date VP, Development Operations _________________________________________________________________ Ellen Carey Date Senior Director, Regulatory Affairs The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 3 of 23 Table of Contents Statistical Analysis Plan Approval ................................................................................................................ 2 List of Abbreviations .................................................................................................................................... 5 1. Introduction .............................................................................................................................................. 7 2. Sample Size Determination and Re-Estimation ....................................................................................... 7 3. General Statistical Considerations and Definitions.................................................................................. 7 3.1 General Statistical Considerations ..................................................................................................... 7 3.2 Analysis populations .......................................................................................................................... 8 3.3 Statistical Significance Level ............................................................................................................. 8 3.4 Time Windows ................................................................................................................................... 9 3.5 Handling of Dropouts or Missing data ............................................................................................... 9 3.6 Data Base Lock/Unblinding of Treatment ......................................................................................... 9 4. Patient Disposition ................................................................................................................................... 9 5. Demographics and Other Baseline Characteristics ................................................................................ 10 6. Study Drug, Procedures, and Non-Study Drug Medications ................................................................. 10 6.1 Study Drug ....................................................................................................................................... 10 6.2 Non-Study Drug Medications .......................................................................................................... 11 6.3 Procedures ........................................................................................................................................ 12 7. Analysis of Study Endpoints .................................................................................................................. 12 7.1 Primary Endpoint ............................................................................................................................. 12 7.2 Secondary Endpoints ....................................................................................................................... 13 7.3 Clinical Events Committee .............................................................................................................. 14 7.4 Analysis of Primary and Secondary Endpoints ................................................................................ 14 7.5 Time to Event Analyses ................................................................................................................... 15 7.6 Subgroups ........................................................................................................................................ 15 7.7 P2Y12 Analyses .............................................................................................................................. 16 7.8 GPI Analysis .................................................................................................................................... 16 7.9 Bleeding Components ...................................................................................................................... 17 7.10 Meta Analysis/Exploratory Analyses............................................................................................. 17 8. Safety Analyses...................................................................................................................................... 18 8.1 Laboratory Evaluations .................................................................................................................... 18 8.1.1 Hemoglobin and Hematocrit ..................................................................................................... 18 8.1.2 Creatinine and Creatinine Clearance ......................................................................................... 19 The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 4 of 23 8.1.3 Platelets ..................................................................................................................................... 19 8.1.4 Other Laboratory Values ........................................................................................................... 19 8.2 Adverse Events ................................................................................................................................ 19 9. Interim Analysis ..................................................................................................................................... 20 9.1 Interim Analysis Specifications ....................................................................................................... 20 9.2 Alpha Spending Function ................................................................................................................ 20 9.3 Adaptive Sample Size Calculations ................................................................................................. 21 10. Changes in the Planned Analysis ......................................................................................................... 22 References ............................................................................................................................................... 23 The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 5 of 23 LIST OF ABBREVIATIONS Abbreviation Definition AE Adverse Event ARC Academic Research Consortium CABG Coronary Artery Bypass Grafts CEC Clinical Events Committee CI Confidence Interval CrCl Creatinine Clearance DSMB Data and Safety Monitoring Board dL Decilitre(s) ECG Electrocardiogram eCRF Electronic Case Report Form GCP Good Clinical Practice GUSTO Global Utilisation of Streptokinase and tPA for Occluded Coronary Arteries GPI Glycoprotein IIb/IIIa Inhibitor hrs hours IDR Ischaemia Driven Revascularisation ITT Intent-to-treat IU International unit IV Intravenous IVRS Interactive Voice Response System kg Kilogram(s) LMWH Low Molecular Weight Heparin LOCF Last observation carried forward MDCO The Medicines Company MedDRA Medical Dictionary for Regulatory Activities MI Myocardial Infarction mg Milligram(s) mL Millilitre(s) mm Millimetre(s) The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 6 of 23 Abbreviation Definition min Minute(s) PCI Percutaneous Coronary Intervention PP Per Protocol Population SAE Serious Adverse Event SAP Statistical analysis plan STEMI ST-Segment Elevation Myocardial Infarction STE-ACS ST-Segment Elevation Acute Coronary Syndrome SD Standard Deviation TEAE Treatment-emergent Adverse Event TIMI Thrombolysis in Myocardial Infarction UFH Unfractionated Heparin The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 7 of 23 1. Introduction This analysis plan provides a description of the strategy, rationale, and statistical methodology to be used to assess the results for the EUROMAX trial. The purpose of the Statistical Analysis Plan is to ensure the credibility of the study findings by pre-specifying the statistical approaches and processes to the analysis of the study data prior to unblinding the randomization code. EUROMAX is a multi-centre, multi-national, prospective, randomized, open-label study to compare bivalirudin to current therapies in patients with STE-ACS presenting either via ambulance or to centres where PCI is not performed, that are intended for a primary PCI management strategy. The planned sample size is 2200 patients. Patients that qualify for entry into the study are randomly assigned in a 1:1 ratio to receive either bivalirudin or the standard of care. The primary objective of the trial is to show that, when compared to the control arm of standard of care anti-thrombotic therapies that include unfractionated heparin, LMWH and optional GPI, treatment with bivalirudin will be superior in reducing the composite of death and non-CABGrelated protocol major bleeding at 30 days. 2. Sample Size Determination and Re-Estimation The sample size was determined based on the comparison of the composite primary endpoint of death and non-CABG-related protocol major bleeding at 30 days between patients in the bivalirudin and standard of care arm therapies for the trial population. This assumed the event rates in patients with STE-ACS to be 4.25% in the bivalirudin arm versus 7.0% in the control arm. The sample size of 2200 will provide 80% power at a two-sided alpha level of 0.05 for the primary comparison of death and non-CABG-related protocol major bleeding at 30 days. One interim analysis was performed during the course of the study after approximately 70% of randomized patients had completed the 30 day evaluation and all the primary endpoint events had been adjudicated. The analysis was performed by an independent statistical consulting group. The conditional power was calculated and the sample size was re-evaluated. The results of the interim analysis were reviewed by the Data and Safety Monitoring Board (DSMB) who made recommendations to The Medicines Company (MDCO). The planned interim analysis took place on April 9, 2013. After review of the data on 1562 patients and conditional power analysis, the DSMB recommended to MDCO to continue the EUROMAX study as planned and keep the sample size unchanged. 3. General Statistical Considerations and Definitions 3.1 General Statistical Considerations The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 8 of 23 All statistical analyses and summary information will be generated according to this statistical analysis plan (SAP). Any deviations from this SAP will be documented in the clinical study report. All summaries and statistical analyses will be generated using SAS® version 9.2 or higher. Continuous variables will be summarised using means, standard deviations, medians, interquartile ranges and minimum and maximum values. Categorical variables will be summarised using frequencies and percentages. 3.2 Analysis populations For this trial, the following populations will be defined and used in the analysis and/or presentation of the data. Intent-to-treat (ITT) population: The ITT population is defined as all subjects randomised into the trial. Treatment classification will be based on the randomised treatment regardless of whether the randomized treatment was administered. Patients without informed consent are excluded. Per-protocol (PP) population: The PP population is defined as all subjects randomised into the trial who provided informed consent, received their randomised treatment, and who underwent angiography. Safety population: The safety population is defined as all randomised subjects who provided informed consent and received study drug, and will be classified according to the actual treatment received. If a patient received both study drugs, the first one received after randomization will be used for the classification. The primary and secondary efficacy analyses will be based on the ITT population. Analyses based on the PP populations will be considered secondary and confirmatory. All safety analyses will be performed on the safety population. 3.3 Statistical Significance Level A two-sided overall Type I error rate (alpha) of 0.05 will be used for all statistical analyses. For the analysis of the primary endpoint, the Type I error will be adjusted for the planned 70% efficacy interim analysis using the Peto boundary3. Since the primary objective is to show superiority of bivalirudin over the standard of care, the lower boundary is of little interest and the upper boundary will be considered to be a 1-sided, 0.025 boundary. The nominal alpha at the final analysis will be set at 0.024 if no sample size modification is implemented after the 70% interim analysis. Per the DSMB review on April 9, 2013, it was recommended to continue the EUROMAX study as planned with no increase in sample size. Thus the final nominal alpha will stay at 0.024 for the primary endpoint. For the secondary endpoints, no adjustment will be made for multiple comparisons. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 9 of 23 3.4 Time Windows Baseline: The baseline laboratory value is determined as the value recorded on the baseline visit according to the eCRF. The actual date/time is not used in the calculation. Because these blood draws take place in the ambulance, the time entered on the eCRF was often found to be when the laboratory results were either analyzed or received. In the rare event of 2 baseline records, the last one is used. Time 0, Day 0: Study time 0 is defined as the start of the study drug. Start of study drug is the time of the first bolus dose. For bivalirudin, if the time of the bolus dose is missing but there is a non missing infusion time then that time is used. If the study drug times are missing, the time of randomization is used. All calculated study times are relative to time 0 which occurs on Day 0. Day 7 or Hospital Discharge: either day 7 or hospital discharge, whichever is earliest for each patient is used as the cut off point for inclusion of events for this time point.30 day: Events included in the 30 day evaluation occurred up to/including 35 days. Therefore Day 30=date of study drug + 35. 1 year: Deaths included in the 1 year evaluation occurred up to/including 395 days. 3.5 Handling of Dropouts or Missing data A proportion of patients are expected to be lost to follow-up for various reasons. To account for missing information, the primary analysis will use the last observation carried forward (LOCF) approach and will include all patients in the event rate denominator. The data as observed method is utilized by the time to event analysis and will be supportive. For other analyses, no imputation method will be used to infer missing values unless otherwise stated. 3.6 Data Base Lock/Unblinding of Treatment The follow-up period for the study is 1 year for death and 30 days for all other endpoints. The database will be locked after all enrolled patients have completed their 30 day visit and their data cleaned and adjudicated. This will occur before all patients have completed the 1 year follow-up for evaluation of the 1 year mortality endpoint. Before locking the database, the tables, figures, and listings will be done using scrambled/dummy treatment codes and reviewed by data management, statistics and medical. After the 30 day database lock the data will then be unblinded and analyzed using the actual randomized treatments excluding the 1 year death endpoint. The study will continue until all patients have completed their 1 year follow-up and a second database lock will then occur to include the 1 year death endpoint. 4. Patient Disposition The number of patients randomized and within each study population (ITT, PP and Safety) will be summarized. The number of patients in the ITT population will be listed by site and country. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 10 of 23 A patient will be considered to have completed the 30 day portion of the study if any of the following conditions is met: The 30 day visit occurred at day 25 or later The 1 year follow-up was completed The patient had an endpoint event The patient was lost to follow-up ≥ 25 days The patient withdrew consent but allowed data from after 25 days to be used The reasons for failure to complete the 30 days in the study will be summarized as follows: Day 30 visit < 25 days Lost to follow-up < 25 days withdrew consent but allow data to be used prior to date of withdrawn consent and date is <25 days missing 30 day visit with no 1 year or end of study eCRF page missing 30 day visit, end of study status not completed due to other reasons missing 30 day visit, end of study status not completed due to physician decision A summary of all information collected on the end of study page of the eCRF will be summarized by treatment group. 5. Demographics and Other Baseline Characteristics All demographic variables collected on the eCRF will be summarized by treatment group for the ITT, PP and safety populations. Age will be summarized as a continuous variable and also categorized into greater than 65 years and also greater than 75 years. The planned treatment and final diagnosis will be summarized. The specific diagnosis will be listed for diagnoses chosen as other than STEMI, Myo/pericarditis, pulmonary embolism or Tako-tsubo Cardiomyopathy. All information collected on the medical history page of the eCRF will be summarized by treatment group for the ITT, PP and safety populations. 6. Study Drug, Procedures, and Non-Study Drug Medications 6.1 Study Drug The following will be summarized for bivalirudin: bolus dose weight adjusted mg/kg The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 11 of 23 first infusion dose rate weight adjusted mg/kg/hr second infusion dose rate weight adjusted mg/kg/hr total infusion duration minutes: For each infusion dose calculate the difference between the start and stop time. The total is then the sum of each duration. Durations will also be presented for each infusion separately. total dose mg: To convert the infusion dose to mg, the duration of each separate infusion is calculated (stop time – start time) in hours and multiplied by the infusion dose in mg/hr. All bolus and infusion doses are then added together. pre PCI infusion duration minutes: the time from the start of the infusion to the start of the PCI duration from start of PCI minutes: the time from the start of the PCI to the end of the last infusion. This will be summarized as a continuous variable and also broken into the following categories: <3 hrs, 3-3.5 hrs, 3.5-4 hrs. 4-4.5 hrs, 4.5-5 hrs, >5 hrs. It will also be presented separately for patients with only 1 infusion. Note: the stop time of the PCI was not collected on the eCRF. For the standard of care treatment arm the following will be summarized: first bolus dose weight adjusted IU/kg (UFH) or mg/kg (LMWH) second bolus dose weight adjusted IU/kg (UFH) or mg/kg (LMWH) infusion dose rate weight adjusted IU/kg/hr infusion duration minutes total dose UFH in IU: To convert the infusion dose to IU, the duration of the infusion is calculated (stop time – start time) in hours and multiplied by the infusion dose in IU/hr. All bolus and infusion doses are then added together. total dose LMWH in mg bolus to PCI start minutes: the time from the start of the bolus to the start of the PCI type of bolus given: either UFH or LMWH 6.2 Non-Study Drug Medications All non-study drug medication information collected on the eCRF will be summarized by treatment for the ITT population. This includes aspirin, P2Y12 inhibitors loading and maintenance doses, coumarin type drugs, UFH, LMWH, fondaparinux and GPI. The discharge medications will also be summarized. The following 3 parameters will be summarized by site and country: The number of patients given a GPI in the standard of care arm (for bailout and routine use combined) The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 12 of 23 The number of patients with a radial arterial access The number of patients given a prasugrel loading dose The number of patients given a ticagrelor loading dose 6.3 Procedures All data captured on the eCRF for the angiography, PCI and the occurrence of CABG will be summarized by treatment for the ITT population. The start of the study drug until both the start of the angiography and the PCI will be calculated. The time from arrival in Cath Lab at primary PCI capable hospital to the start of the PCI will also be calculated. The length of the hospital stay will be calculated as the time from the arrival in the Cath Lab until the hospital discharge date. 7. Analysis of Study Endpoints 7.1 Primary Endpoint The primary endpoint will be the composite of death and non-CABG-related protocol major bleeding evaluated at 30 days. Study endpoints will be adjudicated by the Clinical Events Committee (CEC) which is blinded to the treatment assignment. Death: will be defined as death from any cause at any time. In addition, the cause of death (cardiac versus non-cardiac) will be adjudicated. Cardiac death is defined as death due to any of the following: Acute myocardial infarction Heart failure Cardiac perforation/pericardial tamponade Arrhythmia or conduction abnormality Cerebrovascular accident suspected of being related to the index procedure (PCI/CABG) Death due to complication of the index procedure (PCI/CABG) Any death in which a cardiac cause cannot be excluded Non-cardiac death is defined as a death not due to cardiac causes (as defined above), including bleeding-related death. All Bleeding events: will be characterised as related or unrelated to surgery (CABG). Protocol Major Bleeding: will be characterised as unrelated to surgery (CABG) and defined as any one of the following: Intracranial Retroperitoneal The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 13 of 23 Intraocular Access site haemorrhage requiring radiological or surgical intervention Reduction in haemoglobin concentration of >4g/dL (2.5 mmol/L) without an overt source of bleeding Reduction in haemoglobin concentration of >3g/dL (1.8 mmol/L) with an overt source of bleeding Re-intervention for bleeding Use of any blood product transfusion 7.2 Secondary Endpoints The time point for the secondary endpoints is specified as events occurring within 30 days with death also at 1 year. However, all endpoints will also be presented at day 7 or hospital discharge, whichever occurs first. Key Secondary Endpoint The composite endpoint of death, MI, or protocol major bleed at 30 days is the key secondary endpoint. Secondary Endpoints: Death Major Bleeding (protocol, TIMI and GUSTO) Minor Bleeding (protocol, TIMI and GUSTO) Re-infarction (MI) IDR Stent thrombosis (ARC definition) Stroke Incidence of thrombocytopenia Composite Secondary Endpoints Specified in the Protocol: Death or re-infarction (MI) Death, re-infarction (MI) or IDR Death, re-infarction (MI) or non-CABG-related Protocol Major Bleeding The following additional Composite Endpoints will also be derived: The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 14 of 23 Death, MI, IDR or Stroke MI, IDR or Stent thrombosis MI or IDR with no Stent thrombosis Death, MI, IDR or Protocol Major Bleed Death, Q-wave MI or Protocol Major Bleed Death, MI, IDR, Stroke or Protocol Major Bleed A subject is defined to have a composite event if the subject experiences at least 1 of the components. If the subject does not have any of the components, then they did not have the composite endpoint. If a patient has more than 1 of the components, they are only counted once in the determination of the total number of patients experiencing the composite endpoint. Death will be separated into cardiovascular and non cardiovascular and all composite endpoints containing death will also be calculated using just cardiovascular death. IDR will be separated into PCI and CABG. MI will be further delineated as Q-wave and non-Q wave. Stroke will be quantified as ischemic, hemorrhagic or other. Stent thrombosis will be classified as either definite or probable and as acute or subacute. 7.3 Clinical Events Committee All study endpoints except thrombocytopenia will be adjudicated by the independent Clinical Events Committee which is blinded to the treatment assignment. CEC adjudicated events will be used in the primary and secondary analyses. The CEC determined if the event met the criteria of the endpoint definition, specified the applicable subcategories (i.e. Q-wave or non-Q wave for MIs, major or minor for bleeds) and entered a date & time for the event. The CEC consists of 2 members. The MDCO Biostatistics group was responsible for the triggering program which flagged events to be adjudicated and for comparing the 2 adjudicator’s results for agreement. When the adjudicator’s results did not match, they met to resolve the discrepancy. Agreement was required for all event subcategories and time windows. Time windows for matching were 0-35 days for all events with death having an additional window of 36-395 days. Stent thrombosis was further divided into 0-24 hours for acute and 24 hours to 35 days for sub acute. The exact date/time did not have to match, just the time window. The adjudicated date/time as determined by the CEC chair was the one used in the analysis. Since there was a small possibility data might have changed after an event was adjudicated, any event that was no longer flagged by the trigger program, but had been positively adjudicated, was sent back to be re-adjudicated. Data that changed, but were still flagged, produced a new triggered event to be adjudicated. 7.4 Analysis of Primary and Secondary Endpoints Event rates for each treatment group and total will be presented along with the relative risk and 95% confidence intervals (CI). P-values will be based on the Chi-square test. For expected cell The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 15 of 23 counts less than 5, Fisher’s Exact test will be used instead and denoted in the table. The primary population will be the ITT population with the PP population as supportive. The denominator for the event rates will be the population sample size. The LOCF approach will be used to impute the missing data for the primary efficacy analysis in the ITT population and the observed-case approach will be supportive. An additional analysis will be done for the stent thrombosis endpoint. Event rates will also be calculated and tested using as the denominator only those patients that received a stent. 7.5 Time to Event Analyses As a supportive analysis time to event analysis will be performed. For composite endpoints the time to event is considered the time of the first event. If a patient did not have an event the follow-up time will be considered censored and derived as follows (applies to all events except death): 1. Use date of the 30 day visit. 2. If 30 day visit is not done but the end of study eCRF page or 1 year visit is completed: If lost to follow-up & date of last known contact/withdrawal≤35 use date of last known contact If lost to follow-up & date of last known contact/withdrawal>35 use day 35 If completed study use day 35 If 1 year visit completed use day 35 3. If 30 day visit is not done & both end of study eCRF page & 1 year visit are missing: find the last date among the following: day 7 visit, any date from bleed or cardiovascular endpoint eCRF pages, hospital discharge date, laboratory date. Follow-up time for death will be 35 days unless the patient is lost to follow-up & the date of last known contact/withdrawal <35 days, then the date of last known contact/withdrawal will be used. The log-rank test will be performed for inference and event rates will be estimated using the Kaplan-Meier method. The Kaplan-Meier curve will be plotted through day 30. 7.6 Subgroups Subgroup analyses will be performed on the following 6 endpoints at 30 days (additional endpoints may be added): Death or Protocol Major Bleeding Death, MI or Protocol Major Bleeding Death, MI or IDR Death, MI, IDR or Protocol Major Bleeding The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 16 of 23 Protocol Major Bleeding Death Subgroup analyses will include, but are not limited to, the following: Age group cut off at 65 Gender Diabetes Previous MI Arterial Access Site More than 1 vessel with stenosis >50% LAD target vessel Stent type – at least 1 DES, all bare metal Killip Class greater than 1 Loading Dose – Clopidogrel, prasugrel, ticagrelor Maintenance Dose – Clopidogrel, prasugrel, ticagrelor Time on drug to angiography cut off at 50 minutes GPI Site defined as a site where 50% or more of the standard of care arm receives a GPI Creatinine Clearance cut off at 60 The chi-square test comparing the 2 treatments and relative risk with 95% CI will be presented for each category of the subgroup. The interaction between the subgroup and the treatment will be tested with logistic regression. 7.7 P2Y12 Analyses For each treatment arm separately, a comparison will be made between the 3 P2Y12 medications – clopidogrel, prasugrel and ticagrelor. Also for each P2Y12 medication, a comparison between the 2 treatment arms will be made. For the day 7 time point the loading medication will be used and for the 30 day time period the maintenance medication will be used. All adjudicated endpoints will be tested with the chi-square test and the relative risk and 95% CI will be presented. 7.8 GPI Analysis The standard of care arm will be broken out into 2 groups based on if they received a GPI. The first group will consist of patients that received heparin plus GPI given as routine. The second group will contain both patients that did not receive GPI plus those patients that got GPI as bailout. These 2 standard of care groups will be compared to the bivalirudin group for all adjudicated endpoints at both the day 7 and 30 day time points. The chi-square test will be used and the relative risk and 95% CI will be presented. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 17 of 23 7.9 Bleeding Components The individual bleeding components as recorded on the eCRF will be summarized at 30 days. For patients with an adjudicated major bleed, the following components will be tabulated: Intracranial Retroperitoneal Intraocular Cardiac tamponade Access site haemorrhage requiring radiological or surgical intervention Clinically overt bleed Drop in haemoglobin or hematocrit Re-operation for bleeding blood transfusion hemodynamic compromise For patients with an adjudicated minor bleed, the following components will be tabulated Gross haematuria Haematemesis Ecchymosis Epistaxis Oozing Drop in haemoglobin or hematocrit Other 7.10 Meta Analysis/Exploratory Analyses The HORIZONS-AMI trial is also a study of bivalirudin in STEMI patients. It is planned to combine the results of the EUROMAX trial with the HORIZONS trial. The EUROMAX study differed from HORIZONS in the following 4 main areas: the bivalirudin dosing - in the ambulance & extending for 4 hours after the end of the PCI the optional use of GPI in the standard of care arm the use of the newer P2Y12 agents (prasugrel and ticagrelor) Participating countries How these differences, including other factors, relate to the trial outcomes will be explored with logistic regression analysis or Cox proportional hazards modeling. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 18 of 23 Since HORIZONS has been previously pooled with the ACUITY and REPLACE 2 trials, EUROMAX may also be combined with these trials. 8. Safety Analyses 8.1 Laboratory Evaluations Table 1 lists the laboratory unit conversion factors that were used. Table 1 Laboratory Unit Conversion factors Unit Lab Parameter From Creatinine Hemoglobin Hematocrit Platelet Troponin I/T mg/dL mmol/L mg/L mol/L gm%, g/dL, gm/dL mmol/L mol/L g/L % L/L, ratio k/L, T/cumm, g/L, x10/mm, x10^3/L, x10^3/mm^3, nl, k/ mm^3 1000/mcl, 1000/ mma^3 x10^9/L, k/cmm, x10^9/L, xThou, Gpt/L, x10^3 /cumm, /L x1012/L ng/mL To Conversion Factor mg/dL mg/dL mg/dL mg/dL g/dL g/dL g/dL g/dL % % k/mm^3 1 11.31 0.1 0.01131 1 1.611 0.001611 0.1 1 100 1 x10^9/L x10^9/L ug/L 0.001 1000 1 Laboratory variables will be analyzed in the same time window they were collected in. That is, the page the value was recorded on in the eCRF is the determination for the time period (baseline, day 7, day 30). Summaries will be based on the safety population. 8.1.1 Hemoglobin and Hematocrit Hemoglobin and hematocrit values at each visit and the change from baseline will be summarized. The number of patients with a drop in haemoglobin greater than 3 g/dL and hematocrit greater than 9% will also be summarized. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 19 of 23 8.1.2 Creatinine and Creatinine Clearance Creatinine values at each visit and the change from baseline will be summarized. The number of patients with an abnormal value at baseline will also be summarized. An estimate of Creatinine Clearance (CrCL) will be calculated using the Cockcroft-Gault formula 𝐶𝑟𝐶𝐿 = (140 − 𝐴𝑔𝑒) 𝑥 𝑀𝑎𝑠𝑠 (𝑖𝑛 𝑘𝑖𝑙𝑜𝑔𝑟𝑎𝑚𝑠) 𝑥 [0.85 𝑖𝑓 𝐹𝑒𝑚𝑎𝑙𝑒] 𝑚𝑔 72 𝑥 𝑆𝑒𝑟𝑢𝑚 𝐶𝑟𝑒𝑎𝑡𝑖𝑛𝑖𝑛𝑒 (𝑖𝑛 ) 𝑑𝐿 The baseline CrCl will be grouped into the following 3 categories of renal insufficiency and summarized. Severe: CrCl < 30 mL/min Moderate : CrCl 30 – 60 mL/min Normal: CrCl > 60 mL/min 8.1.3 Platelets Platelet values at each visit and the change from baseline will be summarized. The number of patients with an abnormal value at baseline will also be summarized. The baseline values will be summarized as less than and greater than 100 k/mm^3. A 50% drop from baseline will also be calculated. Thrombocytopenia will be summarized at day 7 and day 30 as mild (50 – <100 k/mm^3) moderate (20 – <50 k/mm^3) and severe (<20 k/mm^3) for patients without thrombocytopenia (<100 k/mm^3) at baseline. The chi-square test will be used to test thrombocytopenia and the relative risk and 95% CI will be presented. 8.1.4 Other Laboratory Values Creatine Phosphokinase, Creatine Kinase MB Isoenzyme, Troponin T and Troponin I will be summarized at baseline. 8.2 Adverse Events All adverse events (AE) will be listed. All AEs will be coded using the dictionary terms from the MedDRA Adverse Reaction Dictionary. Treatment-emergent AEs (TEAE) are defined as events which occur, or worsen on or after the time of the first study drug. The frequency of TEAEs and serious adverse events (SAE) will be summarized by system organ class, preferred term, relationship to study drug and severity. If more than one event occurred with the same preferred term for the same patient, the patient will be counted only once for that preferred term using the most severe or related occurrence for the summary by severity, or relationship to study drug, respectively. An individual patient will be counted only once for any specific preferred term regardless of the number of times that preferred term occurred for that patient. Similarly, a patient will be counted only once for the overall AE incidence rate, regardless of how many AEs occurred for that patient. Relationship to study drug will be defined as related if possibly related, The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 20 of 23 or definitely related was chosen by the investigator. The number of patients with the study drug withdrawn or the dose reduced due to an AE will be summarized. The following comparisons between treatment groups will be tested using the Chi-square test: Number of patients with at least 1 TEAE Number of patients with at least 1 related AE Number of patients with at least 1 SAE Number of patients with at least 1 related SAE 9. Interim Analysis 9.1 Interim Analysis Specifications The interim analysis will be performed when approximately 70% of the planned sample size of 2200 patients (1540 patients) has adjudicated primary endpoint data. The primary endpoint is a composite of death or non-CABG-related protocol major bleeding evaluated at 30 days after randomisation. The adjudicated values will be used with the ITT population. Stopping the study at the interim analysis due to futility or superiority is not planned. The study will either continue as planned or the sample size may be increased up to a maximum of 5000 patients. 9.2 Alpha Spending Function Group sequential tests will be performed using the Peto boundary [Peto et al, 1976] as specified in the protocol. The calculation of the Peto boundary did not use the exact method and therefore is slightly conservative. Table 2: Peto Boundary Percent of Patients Upper Boundary Nominal Alpha Cumulative Alpha 70% 3.09023 0.001 0.001 100% 1.97737 0.024 0.025 Since the primary objective is to show superiority of bivalirudin over the standard of care, the lower boundary is of little interest and the upper boundary will be considered to be a 1-sided, 2.5% boundary. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 21 of 23 The test statistic is given by Z= 𝑝𝑐 −𝑝𝑡 𝑝 (1−𝑝𝑐 ) 𝑝𝑡(1−𝑝𝑡 ) √ 𝑐 + 𝑛𝑐 𝑛𝑡 where 𝑝𝑐 , 𝑝𝑡 are the endpoint proportions for the control and bivalirudin treatments and 𝑛𝑐 , 𝑛𝑡 are the sample sizes for the control and bivalirudin treatments. 9.3 Adaptive Sample Size Calculations The adaptive sample size calculations are based on the methods proposed in Mehta and Pocock, 2010. The first step of the interim analysis is to calculate the conditional power. The conditional power at the interim analysis is 𝐶𝑃 = 1 − 𝛷 ( 𝑧𝛼 √𝑛2 − 𝑧1 √𝑛1 𝑧1 √𝑛2 − 𝑛1 − ) √𝑛2 − 𝑛1 √𝑛1 Where 𝑧𝛼 is 1.97737 (from Table 1), 𝑧1 is the Z test statistic using the endpoint proportions & sample sizes from the interim analysis, 𝑛1 is the sample size at the interim analysis and 𝑛2 is the planned final sample size of 2200. The conditional power is then evaluated in terms of the following 3 zones – Unfavorable, Promising and Favorable. Unfavorable defined as CP<30%. In this zone the interim results are so disappointing that it is not worth increasing the sample size. For this zone the study will continue to the original 𝑛2 . Promising defined as 30%≤CP<80%. In this zone the interim results are not disappointing, but not good enough to reach the 80% power specified in the protocol design. In this case the sample size is increased to achieve the targeted 80% power subject to not exceeding nmax (nmax = 5000). The new sample size is the minimum of nmax and 𝑛1 + 𝑛̃ such that the increase in sample size is calculated as 𝑛 𝑧𝛼 √𝑛2 − 𝑧1 √𝑛1 √𝑛2 −𝑛1 1 𝑛̃ = 𝑧 21 [ 2 + 𝑧𝛽 ] Where 𝑧𝛼 , 𝑧1 , 𝑛1 , 𝑛2 are as defined above and 𝑧𝛽 is 0.84162 (β=.2). Thus the new adaptive sample size would be 𝑛1 + 𝑛̃ if that is less than nmax. Otherwise it is nmax. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 22 of 23 Favorable defined as CP≥80%. In this zone the interim results are sufficiently favourable that the trial continues to the original 𝑛2 without the need to adaptively increase the trial size. Because the DSMB recommended to continue the EUROMAX study as planned without a sample size increase, the final analysis critical value (alpha level) for the primary endpoint will stay as planned at 0.024 for the one sided test. 10. Changes in the Planned Analysis Two amendments were made to the study protocol. The second amendment was made on April 24, 2012. The Statistical Analysis Plan is developed based on this version of the protocol. No changes were made from the statistical plan specified in Protocol Amendment 2. Any changes to the analysis made after this SAP has been finalized will be documented in the clinical study report. The Medicines Company Final August 22, 2013 Bivalirudin EUROMAX Confidential Statistical Analysis Plan Page 23 of 23 References 1. Gao P,Ware J, Mehta C (2008). Sample size re-estimation for adaptive sequential design in clinical trials. J. Biopharm. Statist., 18(6), 1184-96. 2. Mehta CR and Pocock SJ (2010). Adaptive Increase in Sample Size when Interim Results are Promising: A Practical Guide with Examples. Statistics in Medicine 2000; 00:1-6. 3. Peto R, Pike MC, Armitage P, et al (1976). Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer ;34(6):585-612. The Medicines Company Final August 22, 2013