Using computer algebra for developmental modeling
advertisement

Signal Transduction, Cellerator,
and The Computable Plant
Bruce E Shapiro, PhD
bshapiro@caltech.edu
http://www.bruce-shapiro.com/cssb
1
Overview
• Cellerator
• Chemical Kinetics
• Signal Transduction Networks
– Modular outlook
– Switches, oscillators, cascades, amplifiers, etc.
– Deterministic vs. Stochastic simulations
• Multicellular systems
– Synchrony, pattern formation
– The Computable Plant project
• Model Inference
2
Cellerator
3
Short Cellerator
Demonstration
4
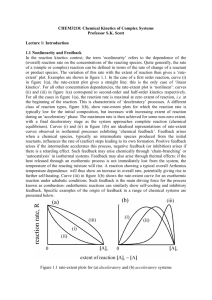
Law of Mass Action
• Canonical form of a chemical reaction:
nR
s x
i1 Ri i
•
•
•
•
•
k
nP
s y
i1 Pi i
Ri,Pi: Reactants, Products
sPi,sRi: Stoichiometry
k: Rate Constant
k3
2HBrO 2 2Ce
Example: BrO 3 HBrO 2
Law of Mass Action: The rate of the reaction is
proportional to the product of the concentrations
of the
reactants.
5
Law of Mass Action (2)
• Formal statement (for a single reaction):
nR
d[X]
sRi
(sPX sR X ) [Ri ]
dt
i1
• Interpretation
k
3
BrO
HBrO
2HBrO 2 2Ce
3
2
of
d
[BrO 3 ] (0 1)k3 [BrO 3 ][HBrO 2 ] k3 [BrO 3 ][HBrO 2 ]
dt
d
[HBrO 2 ] (2 1)k3 [BrO 3 ][HBrO 2 ] k3 [BrO 3 ][HBrO 2 ]
dt
d
[Ce] (2 0)k3 [BrO 3 ][HBrO 2 ] 2k3 [BrO 3 ][HBrO 2 ]
dt
6
Law of Mass Action (3)
• Add rates for multiple reactions
k1
BrO Br
HBrO
HOBr
3
2
k2
“Oregonator”
HBrO 2 Br
2HOBr
k3
BrO 3 HBrO 2 2HBrO 2 2Ce
k4
2HBrO 2 BrO 3 HOBr
k5
Ce Br
d[HBrO 2 ]
k1[BrO 3 ][Br] - k 2 [HBrO 2 ][Br]
dt
+ k 3 [BrO 3 ][HBrO 2 ] - k 4 [HBrO 2 ]2
7
Cellerator Input for Oregonator
Rate Constants
stn={{BrO3+BrHBrO2+HOBr, k1},
{HBrO2+Br2*HOBr, k2},
{BrO3+HBrO2HBrO2+2*Ce, k3},
{2*HBrO2 BrO3+HOBr, k4},
{CeBr, k5}};
interpret[stn, frozen {BrO3}];
Stoichiometry
Hold BrO3
concentration Fixed
8
Cellerator Output for Oregonator
List of Differential Equations and Variables
{
{Br’[t]==-k1*Br[t]*BrO3[t]+k5*Ce[t]k2*Br[t]*HBrO2[t],
Ce’[t]==-k5*Ce[t]+2*k3*BrO3[t]*HBrO2[t],
HBrO2’[t]==k1*Br[t]*BrO3[t]k2*Br[t]*HBrO2[t]
+k3*BrO3[t]*HBrO2[t] -k4*HBrO2[t]^2,
HOBr’[t]==2*k2*Br[t]*HBrO2[t]
+k4*HBrO2[t]2+k1*Br[t]*BrO3[t]},
{Br, Ce, HBrO2, HOBr}
}
9
s= predictTimeCourse[stn,
frozen{BrO3},
timeSpan1500,
rates {k11.3, k2 2*106, k334,
k43000., k50.02,
BrO3[t] .1},
initialConditions{HBrO2.001,
Br.003,Ce.05,BrO3.1};
{{0, 1500,
{{Br InterpolatingFunction[{{0., 1500.}}, <>],
Ce InterpolatingFunction[{{0., 1500.}}, <>],
HBrO2 InterpolatingFunction[{{0., 1500.}}, <>],
HOBr InterpolatingFunction[{{0., 1500.}}, <>]}}}}
Output
Input
Cellerator Simulation
10
Plot Results of Simulation
runPlot[s, plotVariables {Br},
PlotRange { {400, 1400}, {0, 0.002}},
TextStyle {FontFamily -> Times, FontSize -> 24},
PlotLabel "Br Concentration"];
Optional
Input
11
Basic Syntax
network = {{reaction, rate constants},
{reaction, rate constants},...}
modifiers
reaction = reactants arrow products
modifiers
arrow or or
or E
• Format of rate constants varies for different arrows
• Modifiers are optional
• Different rate laws for different arrow/modifier combinations
• We will focus on reaction
• Generate differential equation by entering
interpret[network]
12
Basic Mass Action Reactions
SP
S1 S2
P1 P2
m1S1 m2 S2
S
EP
n1P1 n 2 P2
means S
P
Cellerator Syntax:
{S P, k}
{S1 S2 P1 P2 ,k}
{m1S1 m2 S2 n1P1 n 2 P2
{S E P ,k1,k 2 }
and P
S
, k}
We will generally omit explicitly writing the rate constants in the remainder of this
presentation.
13
Catalytic Mass Action Reactions
S C P C
becomes
C
S P
S + C SC
S CE SC P C or SC S + C
SC P + C
S + C SC
SC S + C
S CE SC P C
SC P + C
or
P RE PR S R
P + R PR
PR P + R
PR S + R
becomes
C
SE P
becomes
C
SE P
R
14
Cascades
A1 A2 A3
means A1 A2 , A2 A3 ,
A1E A2 E A3 E
means A1E A2 , A2 E A3 ,
C
A1 A2 A3
C1 ,C 2 ,...
A1 A2 A3
C
A1E A2 E A3
C1 ,C 2 ,...
A1E A2 E A3
F
A1E A2 E A3
R
C
C
C1
C2
means A1 A2 , A2 A3 ,
means A1 A2 , A2 A3 ,
C
C
means A1E A2 , A2 E A3 ,
C1
C2
means A1E A2 , A2 E A3 ,
F
F
R
R
means A1E A2 , A2 E A3
15
Michaelis-Menten Kinetics
C
a
k
• Catalytic Reaction: SE P (i.e., S C E SCP C)
d
d[S]
d[P]
a[S][C]
d[SC],
k[SC]
• Mass action dt
dt
d[C]
d[SC]
a[S][C] (d k)[SC]
dt
dt
• Steady-state assumption:
d[SC] 0 [SC] a [S][C] E 0 [C]
dt
dk
where E0 is total catalyst (bound + unbound)
16
Michaelis-Menten Kinetics (2)
E0
• Solve for [C]
1 [S]/K M
where KM (d k) /a
• Therefore
d[P]
k[S][C] k[S]E 0
v[S]
k[SC]
KM
K M [S] K M [S]
dt
where v=kE0
d[P] k[S][C]
• If [SC]/ E 0 1 then E 0 [C] hence dt K M [S]
17
Michaelis-Menten in Cellerator
S P (literally, {S P, MM[K,v]})
means
d[P]
v[S]
d[S]
dt
K [S]
dt
C
C
S P (literally, {S P, MM[K,v]})
d[P] v[C][S]
d[S]
means
dt
K [S]
dt
C
C
S P (literally, {S P, MM[a,d,k]})
d[P]
k[C][S]
d[S]
means
dt
(k d) /a [S]
dt
18
Comparison of models
19
GTP: A molecular switch
20
GTP: A reaction schema
R LE RL
RL (GDP)( ) (RL)(GDP)( )
(RL)(GDP)( ) (RL)( ) GDP
(RL)( ) (GTP) (RL)(GTP)( )
(RL)(GTP)( ) (RL) + (GTP)( )
(GTP)( ) (GTP) ( )
GTP X GDP X * +P
GTP X GTP X *
Y Y *
GTP GDP P
GDP (GDP)( )
R * (GDP)( ) (R*)(GDP)( )
21
GTP: Cellerator schema
22
GTP: Cellerator Simulation
23
RASGTP Switch
24
Cascades
25
MAPK: Mitogen Activate Protein Kinase
Heat Shock, Radiation, Chemical,
Inflamatory Stress
Cell Growth and Survival
lab of Jim Woodget, http://kinase.uhnres.utoronto.ca/ 26
MAPK Cascade
S
KKKE KKK
Ph1
KKK
KKE KK E KK
Ph2
KK
KE K E K
Ph 3
Reactions in solution (no scaffold)
27
MAPK in Solution
Kinase Reactions
Phosphatase Reactions
1st Stage
1st Stage
KKK+SKKK-S
KKK-S KKK+S
KKK-SKKK*+S
KKK*+Ph1KKK*-Ph1
KKK*-Ph1 KKK+ Ph1
KKK*- Ph1 KKK*+ Ph1
2nd Stage (1st Phosphate group)
2nd Stage (1st Phosphate group)
KK+KKK*KK-KKK*
KK-KKK*KK+KKK*
KK-KKK*KKK*+KK*
KK*+Ph2KK*-Ph2
KK*- Ph2 KK+ Ph2
KK*- Ph2 KK*+ Ph2
2nd Stage (2nd Phosphate group)
2nd Stage (2nd Phosphate group)
KKK*+KK*KK*-KKK*
KK*-KKK*KKK*+KK*
KK*-KKK*KKK*+KK**
KK**+Ph2KK**-Ph2
KK**- Ph2 KK*+ Ph2
KK**- Ph2 KK**+ Ph2
3rd Stage (1st Phosphate group)
3rd Stage (1st Phosphate group)
K+KK**K-KK**
K-KK**K+KK**
K-KK**KK**+K*
K*+Ph3K*-Ph3
K*- Ph3 K+ Ph3
K*- Ph3 K*+ Ph3
3rd Stage (2nd Phosphate group)
3rd Stage (2nd Phosphate group)
KK**+K*K*-KK**
K*-KK**KK**+K*
K*-KK**KK**+K**
K**+Ph3K**-Ph3
K**- Ph3 K*+ Ph3
K**- Ph3 K**+ Ph3
28
MAPK Cascade on Scaffold
• Scaffold binding significantly increases the rate
of phosphorylation
• Scaffold has 3 slots: one for each kinase
• Each slot can be in different states
– Slot 1: empty, KKK, or KKK* bound
– Slot 2: empty, KK, KK*, or KK** bound
– Slot 3: empty, K, K*, K** bound
• Enter/leave scaffold in any order
• KKK* and either KK or KK* must be bound at
same time produce KK**, etc.
• Number of reactions increases exponentially
with number of slots
29
Effect of Scaffold on Simulations
Number of Reactions
100000
10000
1000
100
Single Phosphorylation
Double Phosphorylation
10
2
3
4
5
6
Number of slots (N)
30
Reactions in MAP Kinase Cascade
• Phosphorylation in Solution
K ai1
j i1
Ki Kij 1 i 1,
Phi
,n 1, j 0,
, a j 1
• Binding to Scaffold
Sp ,
1
, pi , , pn
Kij
S p1 ,
, pi j, , pn
• Phosphorylation in Scaffold
S
S
p1 , , pi1 ja , pi ai , , pn
i1
K S p1 ,
p1 , , pi1 jai1 , pi ai , , pn
S p1 ,
,
, 0,1, , ai , i j
pi
0,1, ,ai , i j
, pi1 j1, pi ai , , pn
, pi1 j1, pi ai , , pn
31
Effect of Scaffold on MAPK
0.8
0.7
100
MAPK ** dt
0
0.6
0.5
0.4
Control*
K4 - K3 complex formation
0.3
0.2
0.1
0
0
Phosphatases in scaffold
Equal rate constants**
0.5
1
1.5
Scaffold Concentration
2
* Control Simulation: k 2/k1=1000, no phosphatases, K4 does not form complex
** k 2/k1=1 (rate constants for phosphorylation steps of MAPK and MAPKKK)
32
Stochastic Comments
• When the number of molecules is small the
continuous approach is unrealistic
– Differential equations describe probabilities and not
concentrations
k
dP[b(t)]
kP[a(t)]
dt
• At intermediate concentrations the continuous
approach has some validity but there will still be
noise due to stochastic effects.
AB
– Langevin Approach:
d[B]
k[A] f (t)
dt
33
Direct Stochastic Algorithm
Gillespie Algorithm (1/3):
• At any given time, determine which reaction is
going to occur next, and modify numbers of
molecules accordingly
Reactions : R1,R2 ,...,RN
Rate Constants : k1,k 2 ,...,kn
Concentrations : X 1, X 2 ,..., X M in volume V
Numbers : Ni X iV
State of system at t : {X 1, X 2 ,..., X M }
# of Distinct Molecular Combinations in Ri : h1,h2 ,...,hN
hi depends combinatorically on the X 1, X 2 ,..., X M
Gillespie DT (1977) J. Phys. Chem. 81: 2340-2361.
34
Gillespie Algorithm (2/3)
Probability that reaction Ri will occur in (t,t + dt) :
Pi (t)dt ai e
t j a j
dt, ai hi ki
Probability that Ri is the next reaction :
Pi Pi (t)dt ai (a1 a2
aN )
Probability that some reaction will occur in ( t,t + dt) :
P(t)dt i Pi (t)dt i ai e
t j a j
dt
35
Gillespie Algorithm (3/3)
Let t=0
While t<tmax {
Calculate all the ai=hiki and a0=Saj
Generate two random numbers r1, r2 on (0, 1)
The time until the next reaction is t=(1/a0)ln(1/r1)
Set t = t + t
Reaction Rj occurs at t, where j satisfies
a1+a2+…+aj-1 < r2a0 ≤ aj+aj+1+…+an
Update the X1,X2,…,Xn to reflect the occurance of
reaction Rj
}
36
Stochastic MAPK Simulation (1/3)
37
Stochastic MAPK Simulation (2/3)
38
Stochastic MAPK Simulation (3/3)
39
Analysis of Multi-step reactions
a
a
a
X L E XL, XL L E XLL, XLL L E XLLL,
d
d
Simplify to:
X nL E Z
a
d
n1
XL
a
L E XLn
d
d
Steady State Solution
d[Z ]
a[X][L]n d[Z ] 0
dt
d[Z ]SS
[X]SS
N [Z ]SS
n
a[L]
N [X][Z ]
[Z ]SS
N[L]n
[L]n (d / a)
Adding steps increases sensitivity
40
Analysis of multi-stage reactions
• Consider two stages of a cascade with
m and n steps
a y [Y]n
a x [X]m
, Z
• Steady State: Y
m
n
K x [X]
K y [Y]
a [X]m n
a y x
n
mn
m
a
a
[X]
K
[X]
x
y x
Z
a [X]m n K K [X]m n a n [X]mn
y
x
x
K y x
m
K x [X]
41
Analysis of multi-stage reactions
• If [X]<<Kx
a x [X]m
ax
m
Y
[X]
K x [X]m K x
n
a x
a y [X]m
n
mn
a y [Y]
a
[X]
K x
y
Z
n
n
n
mn
K y [Y]n
a x
K
K
/
a
[X]
y x
x
m
K y [X]
K x
• Hill exponent is product of m and n
– E.g., a three-step stage followed by a four-step
stage behaves like a 12-step stage
• By incorporating negative feedback can
produce high-gain amplification (see refs).
42
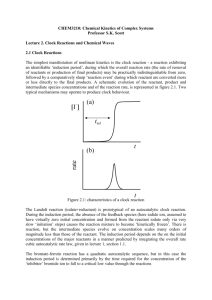
Oscillators in Nature
• Where they occur (to name a few):
–
–
–
–
–
–
Circadian rhythms
Mitotic oscillations
Calcium oscillations
Glycolysis
cAMP
Hormone levels
• How they occur: feedback
– Both negative & positive feedback systems
– Some have feed-forward loops also
43
Negative feedback: canonical model*
dx
dy
S x y,
x y, , , , 0
dt
dt
QuickTime™ and a
TIFF (LZW) decompressor
are needed to see this picture.
Equivalent second order system:0
x 2ax bx S, a ( ) / 2 0, b 0
Characteristic equation: r 2 2ar b 0 r a a2 b
a 2 b stable spiral
a 0 undamped
oscillations
S /b = steady state
a=0.25,b=10,=1,S=5
*Hoffmann et al (2002) Science 298:1241
44
2-species ring oscillator
Y
Y
Y*
X
X X *,Y Y *, X * X ,Y * Y
X*
X
X*
Y*
d[X] v3 (1[X])(1[Y]) v1[X][Y]
dt
1 K M 3 [X]
K M1 [ X]
d[Y] v4 [X](1[Y]) v2 (1[X])[Y]
dt
1 K M 4 [Y]
K M 2 [Y]
X X* 1
Y Y * 1
45
3-species ring oscillator
Z
X
X
Y
Z*
X*
Y*
X X *,Y Y *, Z Z *, X * X ,Y * Y , Z * Z
X*
d[X] v(1[X])(1[Z]) v[X][Z]
dt
1 K M [X]
K M [X]
Y
d[Y] v(1[X])(1[Y]) v[X][Y]
dt
1 K M [Y]
K M [Y]
Y*
d[Z] v(1[Y])(1[Z]) v[Y][Z]
dt
1 K M [Z]
K M [Z]
Z
Z*
46
3-species Ring Oscillator
v=10KM
Robust oscillations
v=KM
Damped oscillations
47
Repressilator
Z
RNA
PZ
Y
RNA
PY
X
PX
RNA
Constructed in E. coli
Elowitz & Leibler,
Nature 403:335 (2000)
48
Repressilator Model Simulations
d[X]
1[PY]n
d[PX]
0 n
k[X],
{[X] [PX]}
n
dt
dt
K [PY]
d[Y]
1[PZ]n
d[PY]
0 n
k[Y],
{[Y] [PY]}
n
dt
dt
K [PZ]
d[Z ]
1[PX]n
d[PZ]
0 n
k[Z],
{[Z] [PZ]}
n
dt
dt
K [PX]
49
Cell Division - Canonical Model
C
MI
M
XI
Goldbeter (1991) PNAS USA, 88:9107
“Minimal” Model of
Cell Division
X
d[C]
0.1[C][X]
0.023 0.00333[C]
dt
0.02 [C]
d[M]
0.5[C](1[M])
0.167[M]
dt
(0.3[C])(1.1[M]) 0.1[M]
d[X] 0.1[M](1[X]) 0.1[X]
dt
1.1[X]
0.1[X]
50
Cell Division - Canonical Model
51
Multi-cellular networks
Intracellular Network
e.g., of mass action, etc.
Transport,
ligand/receptor
interactions, etc
Species xi in
cell j
d j
xi f (x1j ,..., x Nj ) kNbr( j) jk g jk (x1k ,..., x Nk )
dt
kNbr( j) M ijk (xik x kj )
Set of neighbors
of cell j
Connection
matrix
Diffusion Tensor
52
Example - coupled oscillators
n Coupled Oscillators
Two Coupled Oscillators
xi 2 xi jnbr(i) j x j 0
x 2 x y 0
i 1, 2,..., n
y 2 y x 0
Two uncoupled Oscillators
Two Coupled Oscillators, /=.1
53
Example - 105 coupled oscillators
54
Example - 105 coupled oscillators
QuickTime™ and a
Video decompressor
are needed to see this picture.
55
Coupled nonlinear oscillators
C
MI
M
XI
X
XI
M
XI
M
X
M
X
C
MI
X
C
XI
M
XI
MI
C
MI
X
C
MI
C
MI
M
XI
X
Arbitrarily let species X in CMX model diffuse to adjacent cells
56
Coupled CMX Oscillators
d[C j ]
dt
0.023 0.00333[C j ]
d[M j ]
dt
0.1[C j ][X j ]
0.02 [C j ]
0.5[C j ](1[M j ])
(0.3[C j ])(1.1[M j ])
0.167[M j ]
0.1[M j ]
d[X j ] 0.1[M j ](1[X j ]) 0.1[X j ]
DkNbrs( j) ([Xk ] [X j ])
dt
1.1[X j ]
0.1[X j ]
•All oscillating at same frequency
•But different phases
•What happens if you have 105 coupled oscillators with
random phase shifts?
57
105 CMX Oscillators: uncoupled
58
105 CMX Oscillators: uncoupled
QuickTime™ and a
Video decompressor
are needed to see this picture.
59
105 CMX Oscillators: low coupling
D 0.01
60
105 CMX Oscillators: higher coupling
D 0.1
61
105 CMX Oscillators: higher coupling
QuickTime™ and a
Video decompressor
are needed to see this picture.
D 0.1
62
105 CMX Oscillators: Random Period
Uncoupled motion
63
105 CMX Oscillators: Random Period
D 0.1
64
105 CMX Oscillators: Random Period
QuickTime™ and a
Animation decompressor
are needed to see this picture.
Uncoupled
QuickTime™ and a
Animation decompressor
are needed to see this picture.
Coupled Oscillators
65
Pattern Formation
Activator-Inhibitor Models
A
A
A
Single Diffusing Species
X Y
X Y
Two Diffusing Species
•Self-activating (locally)
•X: Activator
•Self-inhibitory (externally)
•Y: Inhibitor
66
Two species pattern formation model
Continuous model*:
d[X]
a b[X] [X]2 /[Y] D1 2 [X]
dt
d[Y]
[X]2 [Y] D2 2 [Y]
dt
Discrete implementation:
{E X j , a, b}, {Y j , 1},
{X j X j Y j , X j }, {2 X j X j , 1/Y j },
{X j E Xi , D1}, {Y j E Yi , D2 }
*See Murray Chapter 14 for detailed analysis
67
Single species pattern formation model
Continuous (logistic) model:
v M [A]nbrs[Anbr ]
d
[A] v[A](1 [A]/K)
dt
[A] K M
Discrete implementation:
v
A[i] E 2A[i], rate constants v, v/K
v /K
A[ i ]
A[ j] , Michaelis constants v M ,K M
Steady State Equation (v=K=vM=KM=1, x=[A])
x
2
0
x(1
x)
x
x
0
or
0
1
x
i xi
i i
1 x
68
Single species pattern formation model
x 0 or 0 1 x 2 i xi
x 1 is a steady state only if all neighbors are at x=0
Suppose that there are nx neighbors in state x and all other
neighbors are in state x=0, 0≤nx6
0 1 x 2 n x x x (1/2)(n x n 2x 4)
Example: case when nx =1 (exactly one neighbor at x, all
others at 0):
x (1/2)(1 5) 0.618034
Question: what other combinations are possible?
69
Single Species Model - 105 Cells
70
Single Species Model - 105 Cells
QuickTime™ and a
Video decompressor
are needed to see this picture.
71
Computable Plant Project
Provide:
•most of our food and
fiber
• all of our paper,
cellulose, rayon
• pharmaceuticals
• feed stock
• waxes
• perfumes
72
Image courtesy of E. M. Meyerowitz, Caltech Division of Biology
Shoot Apical Meristem
growing tip of a plant
Computable Plant Project
• NSF (USA) Frontiers in Integrative Biological
Research (FIBR) Program
• S/W Architecture: Production-scale model inference
– Models formulated as cellerator reactions or SBML
– C++ simulation code autogenerated from models
– Mathematical framework combining transcriptional
regulation, signal transduction, and dynamical mechanical
models
– Simulation engine including standard numerical solvers and
plot capability
– Nonlinear optimization and parameter estimation
– ad hoc image processing and data mining tools
• Image Acquisition
– Dedicated Zeiss LSM 510 meta upright laser scanning
confocal microscope.
• http://www.computableplant.org
73
Computable Plant Project
Experiments
Mathematical Model
Generation
Data Mining
Local
Data Sets
Online
Optimization
User
Regulations &
Reactions
Solvers
Plotters
Automated
Code Generation
Simulation
74
Model Organism
Arabidopsis Thaliana
75
Cell Identification in image z-stack
76
Identification of Cell Birth
77
Image courtesy of E. M. Meyerowitz, Caltech Division of Biology
Shoot Apical Meristem
78
Meristem Pattern Maintenance Model
79
Simulation of Meristem Growth
QuickTime™ and a
Video decompressor
are needed to see this picture.
80
Systems Biology Markup Language
http://sbml.org
libsbml (C++)
MathSBML
(Mathematica)
81
The Standard Paradigm of Biology
RNA
82
Microarrays Produce a lot of data!
Affymetrix GeneChip®
microarray.
Images courtesy of
Affymetrix.
83
RNA Fragments are Selectively Sticky
84
Affymetrix GeneChip® Scanner 3000
with workstation
Data from an experiment showing the
expression of thousands of genes on a
single GeneChip® probe array.
Images courtesy of Affymetrix.
85
Model Inference: Fitting A Model to Data
• Cluster to reduce data size
• Use simplest possible mathematical
possible to determine connectivity
– Fit parameters with some optimization
process: simulated annealing, least
squares, steepest descent, etc.
– Refine model with biological knowledge
– Refine with better accurate math model
– … and repeat until done …
86
Clustering
87
Time
Data clusters in two dimensions
Concentration at time t2
Plot ([X[t3 1],X[t2],X[t3],…,X[tn]) for every species
y
x
Concentration at time t1
88
Concentration at time t2
Data clusters in two dimensions
y
x
Concentration at time t1
89
Signal Transduction Network
Clusters (may) correspond
to functional modules
2
1
T00
T10
T20
T30
T40
T01 T02
T11
T21 T22
T31 T32
T41 T42
0: Output
T12
T03 T04
T13 T14
T23 T24
T33 T34
T43 T44
3
4: Input
90
Approximation Models
• Linear
d[X]i n
M ij [X] j
dt
j1
• S-Systems (Savageau)
d[X]i
cij
cij
t i dt ki j [X] j ki j [X] j
• Generalized Mass Action
d[X]i
ciaj
ciaj
ti
a kia j [X] j a kia j [X] j
dt
91
Approximation Models
• Generalized Continuous Sigma-Pi Networks
d[X]i
ti
g(ui h j )
dt
ui j k Mˆ ijk [X] j [X]k j M jk [X] j
1
x
g(x) 1
2
2
1 x
92
Approximation Models
• Recurrent Artificial Neural Networks
ti
d[X]i
g(ui hi ) i [X]i , ui j M ij [X] j
dt
• Recurrent Artificial Neural Networks with
controlled degradation
d[X]i
ti
g(ui hi ) i [X]i g(uˆi hˆi )
dt
ui j M ij [X] j , uˆi j Mˆ ij [X] j
93
Approximation Models
• Recurrent Artificial Neural Networks with
biochemical knowledge about some species
ti
d[X]i
d[X]i
g(ui hi ) i [X]i t i
dt
dt Cellerator
ui j M ij [X] j
Known or hypothesized interactions due
to mass action, Michaelis-Menten, or
other reactions (A priori knowledge or
assumptions)
94
Approximation Models
• Multicellular Artificial Neural Networks with
biochemical knowledge about some species:
d[X]ia
ta
g(uai ha ) a [X]ia a [X]ia a [X]ia,ext
dt
d[X]i
Resources
t i
dt Cellerator
Diffusion
uai b M ab [X]ib
j ij
Geometric
Connections
j
j
i ˜ (1)
(2)
ˆ
˜
M
[X]
[X]
M
M
[X]
b ab b c c ac b cb b
Lower index: species; Upper Index: Cell
95
Stripe Formation in Drosophila
Dashes- Observations
Solid - Model
Reinitz, Sharp, Mjolsness
Exper. Zoo. 271:47-56 (1995)
96
J Exp Zoology 271:47-56
Patterson, JT Studies in the genetics of drosophila, University of Texas Press (1943);
http://flybase.bio.indiana.edu:82/anatomy/Drosophila
Observed
Some Important Meetings
• ISMB-2004, Scotland, ≈ 30 July 04
Intelligent Systems in Molecular Biology
2003: Australia; 2005: US; 2006:Brazil
• ICSB-2004, Heidelberg, Oct 04
International Conference on Systems Biology
SBML Forum held as satellite meeting
2003:US; 2002:Sweden; 2001:US; 2000: Japan
• PSB-2005, Hawaii, Jan 05
Pacific Symposium on Biocomputing
• RECOMB, Spring 05 Research in Computational Molecular Biology
• BGRS-04, July 04, Semiannually in Novosibirsk
Bioinformatics of Genome Regulation and Structure
• Satellite meetings of many major biology and computer science
meetings: SIAM, ACB, IEEE, ASCB (US), Neuroscience, IBRO,..
97
Collaborators
• Cellerator
– Eric Mjolsness, U. California, Irvine (Computer )
– Andre Levchenko, Johns Hopkins (Bioengineering)
• Computable Plant - Eric Mjolsness, PI
–
–
–
–
–
•
Elliot Meyerowitz, Caltech (Biology)
Venu Reddy, Caltech (Biology)
Marcus Heisler, Caltech (Biology)
Henrik Jonsson, Lund, Sweden (Physics)
Victoria Gor, JPL (Machine Learning)
SBML (John Doyle, PI, Caltech; H. Kitano, Japan)
– Mike Hucka, Caltech (Control & Dynamical Systems)
– Andrew Finney, University of Hertfordshire, UK
98