Confirmation of registration with College Board

AP Biology Summer Work
Mrs. Lacey
Stephine.Lacey@littlerockchristian.com
Introduction
Welcome to AP Biology. The study of life science is an interesting way to see the glory of God in both the details and the grandeur of God’s creative work. Using your textbook, you will review basic Chemistry in chapters 2 and 3. Some basic chemistry facts are necessary to master in order to understand the activities of life at the cellular level. As you work through the written and laboratory assignments, you are welcome to contact me via email with questions. Each section of the summer assignment has a specific due date, please be aware of the fact that you can start the year with easy points if you follow through.
Summer assignment consists of
1.
Personal information emailed to me by July 31, 2015.
2.
Confirmation of registration with College Board emailed to me by July 31, 2015.
3.
Completion of written assignment emailed, or turned in, to me by first day of class.
4.
Completion of lab assignments emailed, or turned in, to me by first day of class.
5.
Laboratory safety contract signed by yourself and a parent by the first day of class.
Class Expectations
AP Biology is designed as a college level course. It is not merely college preparatory; you can earn college credit by successfully completing this course and earning the sufficient grade on the
College Board AP Biology Exam. Through the year, we will review for the exam utilizing the expectations given by the College Board. Because the exam tests at a level higher than expected for a passing grade, the exam is graded using a weighted scale. When you are tested in class using practice exam questions, those tests will be graded using the square root scale. Otherwise, all grades will be given as earned on the typical percentile scale. Assignments that are turned in on the same day will be considered timely unless noted otherwise when assigned. Assignments that are late will be graded and given a grade that is 80% of the grade earned.
Personal Information
To be emailed to me from your school address by July 31, 2015.
1.
Name that you preferred to be called by.
2.
Short explanation of why you elected to take AP Biology.
3.
Short summary of how you like to spend your free time.
4.
Name of favorite movie AND favorite book AND favorite song.
5.
Learning style: a.
How do you feel that you learn best? By reading, hearing, doing, presenting? b.
Where do you do most of your homework? c.
Do you work best in the quiet or with noise? d.
What is your academic strength?
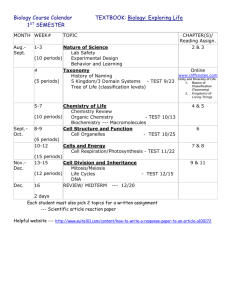
Confirmation of registration with College Board
To be emailed to me from your school address by July 31, 2015.
If you have not already registered, you will need to do so to receive your exam score at the end of this school year. Please do so now, so that you may take advantage of the resources found on the website. https://apstudent.collegeboard.org/home Click on the link “Create one today” .
If you have already registered for a different exam, you do not need to register again. You may forward me any email from the College Board which affirms that you are registered.
To familiarize yourself with the AP exam expectations, watch a summary of the exam: http://www.bozemanscience.com/new-ap-biology-exam-users-guide . Print and complete the review worksheet found on the same page. Turn in completed worksheet with the remainder of the written assignments.
Summer Written Assignment
AP Biology
Mrs. Lacey
Stephine.Lacey@littlerockchristian.com
Read Chapters 2 and 3 of your textbook.
Chapter 2 is a review of basic chemistry concepts. Understanding chemistry is necessary to understanding life science at the molecular level.
1. Referring to the period table, provide the atomic number, element’s symbol, name and mass for the elements essential to life: oxygen, carbon, hydrogen, nitrogen, and phosphorus.
2. For each of the elements above, draw an atom which reflects the number of protons, neutrons, and electrons of each. Label each atom with its symbol. Draw the number of electron shells as appropriate. In the blank below each atom, provide the valence of the element.
Valence _______ Valence _______
2. Continued
Valence _____
Valence _____
Valence _____
Valence _____
3. Research the chemical composition of the human body on the internet. Create a pie chart or bar graph to illustrate the chemical composition of the human body.
4. There are 3 types of chemical bonds: Covalent, Ionic and a category called “Weak Bonds”.
Define each bond below: a. Nonpolar covalent bonds. b. Polar covalent bonds. c. Ionic bonds. d. Hydrogen bonds. e. Van der Waals interactions.
5. After reading the corresponding section in your textbook, watch the video explaining the properties of water that make it the perfect molecule that sustains life. http://www.bozemanscience.com/water-a-polar-molecule
Based on the properties of water, explain the molecular movement of water from the root of a plant to the leaves of its branches.
Based on the properties of water, explain how freezing temperatures will kill the green leaves of a plant.
Chapter 3 introduces the molecules that we need to know to understand the mechanics of life.
You will need to learn the vocabulary of organic molecules along with their structures.
6. Why does the valence of carbon make it the key element of life?
7.Explain the process of dehydration, and its reverse, hydrolysis, by both written statements AND draw a diagram.
8. Carbohydrates are the first important class of biological molecules. Create a chart that summarizes the carbohydrates by name, important chemical structure(s), and use(s) by living organisms.
9. What chemical reaction occurs when two monosaccharides are joined to form a disaccharide?
(An explanation can be found at http://www.biologyalive.com/life/classes/apbiology/documents/Unit%205/media5/05_05Disacchar ides_A.swf
)
10. Lipids are the second important biological molecules. What common trait do all lipids share?
11. One type of lipids are fats. (Additional explanation of fats can be found at http://www.biologyalive.com/life/classes/apbiology/documents/Unit%205/media5/05_11Fats_A.sw
f .) a) The basic structure of a fat is a ______________ molecule plus
___________________. b) If a fat has three fatty acids, it is called a _______________________. c) The difference between a saturated and unsaturated tricylglycerol is
12. A second type of lipids are phospholipids. A phospholipid differs from a fat in what way?
13. A third class of biological macromolecules is proteins. Proteins are made of amino acids linked together. Recreate the representation of the structure of an amino acid found on page 52.
14. Which two portions of an amino acid (drawn on previous page) differs for each of the 20 amino acids?
15. What chemical reaction occurs to join amino acids to make a polypeptide?
16. The structure of a protein (or polypeptide) is defined at four different levels. (Protein structure is explained further at http://www.biologyalive.com/life/index-2.html
. Scroll down through the Unit 5 table of contents until you reach “The Structure and Function of
Macromolecules. Scroll further until you get to “Protein Structure Intro”. There is one slide for each of the four levels of structure.) Create a chart that identifies each level, the shape described by the level, and the chemical bonding which creates the structure.
Summer Laboratory Assignments
AP Biology
Mrs. Lacey
Stephine.Lacey@littlerockchristian.com
The Relative Concentrations of Hydrogen Ions in Various Substances
Understanding the fundamentals of acids and bases is necessary to understand the chemical reactions of biology. Acids, bases and the pH scale are explained in Chapter 2, pages 34-36 in your textbook. This lab will illustrate the effects of acids and bases. Conduct the lab work, complete the attached worksheet and answer the following questions which app ly the information.
NOTES: a.
In order to prove that you actually completed the experiment, I need your parent’s confirmation at the bottom of this page, or a picture of you conducting the experiment emailed to me. You may do this experiment with classmates. b.
If you need further explanation of acid/base chemistry, read http://www.visionlearning.com/en/library/Chemistry/1/Acids-and-Bases/58 c.
100 ml of liquid equals approximately ½ cup.
Follow up questions from lab:
1.Before you added any additional substance, what concentration of H+ could you assume that the water had at room temperature?
2.When you added the cabbage water one of the acids, what happened to the concentration of
H+? Why?
3.When you added the cabbage water to one of the bases, what happened to the concentration of
OH-? Why?
4. Create a diagram or graph that illustrates the relative concentrations of H+ and OH- of all seven substances you tested.
I confirm that my student, ________________________, performed the laboratory work as assigned.
______________________________
Signature
_________________________________
Printed Name