How Sugar and Salt Affect the Boiling Temperature of Water
advertisement

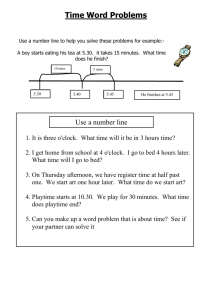
How Sugar and Salt Affect the Boiling Temperature of Water GROUP 4 TLSAMP SUMMER SCIENCE ACADEMY GROUP 4 MEMBERS 1. ASHLEY ARMSTRONG 2. JOHN COLLINS 3. COREY TAYLOR HYPOTHESIS: WE BELIEVE WATER THAT CONTAINS MORE SALT OR SUGAR WILL TAKE THE LEAST AMOUNT OF TIME TO COME TO A BOIL. OBJECTIVES: To Discover How Salt Affects the Boiling Point of Water. Materials Used: Salt Sugar Water (100 ml) Beaker Thermometer Brunson Burner Methods: We used a Brunson burner to boil several amounts of sugar and salt and recorded the temperature and the amount of time each one took to start boiling. We used a hot plate to boil several amounts of sugar and salt and recorded the temperature and the amount of time each one took to start boiling. Data 0 grams 2 grams 4 grams 8 grams 10 grams Salt Group 1 6 mins 4 mins 6 mins 4 mins 5 mins Group 2 4 mins 24 sec 5 mins 8 sec 4 mins 24 sec 4 mins 43 sec 4 mins 50 sec Standard Diviation Sugar 4.280446498 Salt 0.843274043 Sugar Group 3 9 mins 7 mins 7 mins 10 mins 16 mins Group 4 16 mins 13 mins 11 mins 10 mins 20 mins Average Boiling Time Salt Sugar 0 grams 5 12.5 2 grams 4.5 10 4 grams 5 9 8 grams 4 10 10 grams 4.5 18 RESULTS Average Boiling Time 18 16 14 12 10 8 6 4 2 0 Average Boiling Time Salt Average Boiling Time Sugar 0 grams2 grams4 grams8 grams 10 grams CONCLUSION The salt boiled faster then the sugar, and the water that contained more salt boiled quicker than the sugar. The water that contained more salt boiled faster. The more sugar the water contained the slower it boiled.