Questioning Dogma in Microbiology: New Rules and Practice
advertisement

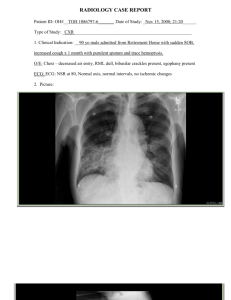
Department of Department of Pathology: Department of Pathology: Department of Pathology: Department Pathology: of Pathology: Questioning Dogmas in Microbiology Paul C. Schreckenberger, Ph.D Director, Clinical Microbiology Lab University of Illinois at Chicago pschreck@uic.edu The University of Illinois at Chicago Medical Center – Urban Medical Ctr. – 450 Bed Teaching Hospital – 400K Ambulatory Visits – 43 Ancillary Departments Broth Culture • RULE: eliminate back-up broths except for Tissue and CSF with shunts -? • References: – Morris AJ et al: JCM 33:161, 1995 – Derby P et al: JCM 35:1101, 1997 – Silletti RP et al: JCM 35:2003, 1997 – Sturgis CD et al. AJCP 108:217, 1997 - CSF – Meredith FT et al. JCM 35:3109, 1997 - CSF – Dunbar SA et al. JCM 36:1617, 1998 - CSF Broth Culture • Savings at UICMC - 1998 Direct Cost Savings Wounds - 3084 x .32 (EB) = $ 990 CSF - 3114 x .32 (EB) = 996 Body fluid - 1905 x .32 (EB) = 610 Stools - 1013 x .45 (GN, HE) = 456 $3,052 Broth Culture • Savings at UICMC - 1998 Indirect Cost Savings Did not have to set up or exam 9116 broth tubes Did not report bogus findings leading to additional testing or therapy of patients Screening Sputum • RULE: perform Gram stain and evaluate under LPF (10 x). Reject if >10 SEC/LPF, unless also see a predominant field of WBCs assoc. with single morphotype of bacteria Screening Sputum • Cancel Culture, Charge for Gram Stain only • DON’T REQUEST REPEAT CULTURE: Add Comment: “specimen contaminated with epithelial cells represents oropharyngeal contamination further processing would yield potentially misleading results.” Screening Sputum • References for using criteria of >10 SEC to reject sputum: 1. Murray PR, Washington II JA: Mayo Clinic Proc. 50:339-344, 1975 2. Wong LK et al: JCM 16:627-631, 1982 Screening Sputum Sputum Quality Indicator UICMC • Using criteria of >25 SEC/LPF – Rejected 20% (range 8-33%) - 1/91-6/92 – QA monitor 12/95 showed rejection rate of 8% • Using criteria of >10 SEC/LPF – Rejected 39.4% (range 32-47%) - 1/96-5/97 – Current Rate 35.4% (range 29-53) - 1/99-12/99 Number of Respiratory Specimens Accepted for Culture 1800 1600 1400 1200 1000 Sputum Bronch Trach 800 600 400 200 0 FY 94 FY 95 FY 96 FY 97 FY 98 FY 99 Screening Endotrachs Endotrach Quality Indicator UICMC • Using same criteria as sputum screen (>10 SEC) – Reject avg. of 4.1% endotrach specimens – For FY 98 rejected only 51 specimens • Using criteria of >10 SEC + NOS on Gram stain – Reject avg. of 25% of endotrach specimens – For FY 98 would have rejected 372 specimens Screening Endotrachs • RULE: specimens with >10 SEC/LPF, or no organisms seen on Gram stain (or yeast only) are not cultured • Reference: – Morris AJ et al: JCM 31:1027, 1993 – Zaidi AK, Reller LB : JCM 34:352, 1996 – Rand KH: Diagn Micro Infect Dis 27:55, 1997 – Gilligan PH: Clin Micro Newsl 21:44, 1999 Endotrach Screening Accept Reject SEC Reject LOC 150 100 50 0 Aug- Sep- Oct- Nov- Dec- Jan- Feb- Mar- Apr99 99 99 99 99 00 00 00 00 Assessing the Quality of Sputum Specimens for AFB Culture • Rejection criteria applied to bacterial cultures based on presence of SEC should not be applied to specimens for AFB culture – Curion CJ et al:JCM 5:381, 1977 – Havlik D, Wood GL: Lab Med 26:411, 1995 – Isaac-Renton JL et al: AJCP 84:361, 1986 – Laird AT: JAMA 52:294, 1909 – McCarter YS, Robinson A: AJCP 105:769, 1996 – Pohl AD Keim AC: Lab Med 24:25, 1993 Assessing the Quality of Sputum Specimens for AFB Culture • Rule: Sputum Specimens Containing No PMNs Are Not Routinely Smeared or Cultured for AFB – Laird AT: JAMA 52:294, 1909 – McCarter YS et al: AJCP 105:769, 1996 – Wilson M: AJCP 105:665, 1996 Correlation of PMN to AFB Positivity McCarter and Robinson AJCP 1996 No. Specimens PMN Present PMN Absent Total – 724 665 (91.9) 59 (8.1) AFB smear Pos – 51 AFB culture Pos – 121 47 (92.2) 4 (7.8) 109 (90.1) 12 (9.9) Correlation of PMN to AFB Positivity McCarter and Robinson 1996 • Based on annual volume of 1378 Sputa and absence of PMNs in 8.1%, • Annual savings of $1,802.00 (based on incremental costs of $3.55 for AFB smear and $12.54 for AFB culture) Guidelines for AFB Cultures • General Order: first morning sputum x 3. Accept only one specimen/day • If first three concentrated smears negative 1. Must initiate consult with lab director 2. If patient is symptomatic, lab will accept three more for up to a maximum of six specimens Guidelines for AFB Cultures • Once three smears are positive 1. Stop accepting respiratory cultures for one month to allow time for cultures to grow 2. Smear requests honored any time as direct smears (not concentrated) until three consecutive negatives are received Guidelines for AFB Cultures • Once cultures are positive No new specimens for culture accepted for 1 month after date of positive culture • References for No. of Sputum Necessary: – Cascina A et al: JCM 38:466, 2000 – Nelson SM et al: JCM 36:467-469, 1998 – Divinagracia RM et al. Chest 114:681-684, 1998 – Peterson EM et al: JCM 37:3564-3568, 1999 Distribution of first positive specimen in patients with >3 AFB specimens Nelson S. et al. JCM 36:467, 1998 Collection Order st 1 2nd rd 3 th 4 or later Total Culture Pos 80(67) 33(28) 7(5) 0(0) 120 Smear Pos 41(73) 8(14) 4(7) 3(6) 56 Smear Neg 39(61) 22(34) 3(5) 0(0) 64 AFB Smear Results Among 43 Patients Culture Pos for MTB Peterson et al. JCM 37:3564, 1999 Collection Concentrated Direct Order Smear Pos Smear Pos st 1 31(72.1) 24(55.8) nd 2 4(9.3) 8(18.6) rd 3 3(7.0) 2(4.7) Total 38(88.4) 34(79.1) Guidelines for Release from Isolation • Patient Receiving effective chemotherapy • Clinical Condition is improving • Three consecutive sputum samples, collected on different days are AFB-smear-negative • References : – Telzak EE, et al: Clin Infect Dis 25:666, 1997 – Iseman MD, et al: Clin Infect Dis 25:671, 1997 – MMWR 43(suppl RR-13):1-132, 1994 Stool Cultures • RULE: Restrict culture and O&P exam to outpatients and inpatients admitted <3 days • RULE: Reject fungal culture on stools. Add statement: “Fungal cultures of stool have not been shown to be clinically useful.” • Reference: – Hines J, et al: Clin Infect Dis 23:1292, 1996 Stool Cultures - Additional Rules • Use MacConkey instead of EMB, allows you to screen for Yersinia without using CIN agar • Eliminate enrichment broths except when looking for asymptomatic carriers • Eliminate serotyping of Salmonella and Shigella, report presumptive result based on biochemical ID, send organism to State Health Lab for typing • Place limitations on AST of stool pathogens Guidelines for Submitting Stool for C. difficile • Test should only be requested when following criteria are met: 1. Antibiotic within 2 mos. prior to diarrhea 2. Diarrhea water/profuse: 6 episodes in 36h 3. Absence of other diagnosis for diarrhea Stool for C. Difficile Repeat Testing Criteria • Negative results: Up to 3 stool specimens (not more than 1 per day) tested per patient • Positive results: after a positive test, further testing only performed 7-10 days after completion of therapy Reference: Barenfanger J, Khardori N: Clin Micro Newsl 18:142, 1996 Questioning Dogmas in Microbiology “Nearly all experts agree that by the year 2000 bacterial and viral disease will have been wiped out” -Time Magazine February 25, 1966