Nonmetals - FSU High Energy Physics
advertisement

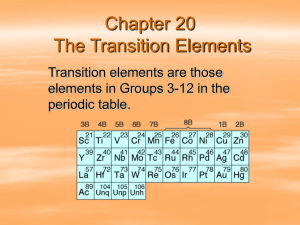
Elements in periodic table With so many elements already found and the possibility of more being discovered, chemists needed a way to organize them. Many systems were tried in order to make some sort of pattern in their properties to match the table. The modern periodic table, based on atomic number and electron configuration, was created primarily by a Russian chemist, Dmitri Ivanovich Mendeleev, and a German physicist, Julius Lothar Meyer, both working independently. They both created similar periodic tables only a few months apart in 1869. Mendeleev created the first periodic table based on atomic weight. He observed that many elements had similar properties, and that they occur periodically, hence the name, periodic table. The modern periodic table elements are ordered by atomic number (= number of protons in the nucleus), not weight. For example, the elements lithium, sodium, potassium, rubidium, and cesium have similar chemical properties. The elements that immediate follow them, beryllium, magnesium, calcium, strontium, and barium, also have similar chemical properties. Elements in Mendeleev's table were arranged in rows called periods. The columns were called groups. Elements of each group had similar properties. Nonmetals Nonmetals: the elements that lack the properties of metals; most often encountered as compounds or mixtures of compounds; some occur in their elemental forms and are very important: e.g. Nitrogen (N2), oxygen (O2), carbon. Properties: almost completely opposite of metals: poor conductors of heat and electricity (except for graphite -- attributed to molecular structure). Many are solids at STP, while many others are gases. All of the group 0 (18) elements (the noble gases--mostly inert) are gases consisting of single atoms. all other gaseous elements, hydrogen, oxygen, nitrogen, fluorine, and chlorine, are diatomic molecules--H2, O2, N2, F2, and Cl2. Bromine and iodine are also diatomic, but bromine is a liquid and iodine is a solid at room temperature. nonmetals also lack the malleability and ductility of the metals -- brittle wide range of reactivity: Flourine is extremely reactive, reacting readily with almost all other elements. Helium is inert and does not react with anything. Metalloids Metalloids are the elements found along the stairstep line that distinguishes metals from non-metals. This line is drawn from between Boron and Aluminum to the border between Polonium and Astatine (exception: Aluminum -- classified under "Other Metals”) list of metalloids: B Boron, Si Silicon, Ge Germanium, As Arsenic, Sb Antimony, Te Tellurium, Po Polonium; properties between those of metals and nonmetals. Generally, metalloids behave as nonmetals, both chemically and physically. They are semiconductors: conduct electricity, but not as well as metals. e.g.: silicon and germanium, used in solid-state electronics; transistors made from semiconductors have reduced the size of electronic components to an almost microscopic level Representative metals The representative metals contain 3 main groups: Alkali Metals: alkali metals alkaline earth metals, post-transitional metals. group IA (1) elements in the table form hydroxides which are strongly basic (e.g., potassium hydroxide--KOH), hence the term "alkaline" have a very high metallic behavior and are good reducing agents. crystallize with a body-centered cubic lattice in which the points are occupied by +1 ions. sea of valence electrons throughout the entire lattice can wander throughout the metal high electrical conductivity and high heat conductivity. high luster is due to the highly mobile electrons of the lattice. Light beams hit the electrons into oscillations, reflecting back electromagnetic energy as light. The softness, malleability, and ductility of the metals are due to the nature of the forces holding the lattice together. Since there is no net force or attraction between the ions, they can be moved from one lattice site to another. The Alkaline Earth Metals The alkaline earth metals are metallic elements in the group IIA (2) of the periodic table. They are called alkaline earth metals because the "earths" of this group, lime (CaO), and magnesia (MgO), give alkaline reactions. All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals arenot found free in nature. They have good metallic properties, including conductivity, reduction ability, luster, softness, malleability, and ductility, but not as well as the alkali metals. Their ions have an oxidation state of +2. Like alkali metals, they form soluble sulfides, but unlike them, they form insoluble carbonates (calcium carbonate -- CaCO3 -- in hard water). List of alkaline earth metals: Be Beryllium Mg Magnesium Ca Calcium Sr Strontium Ba Barium Ra Radium The Post-Transition Metals The post-transition metals: the lower elements of group IIIA (13), IVA (14), and VA (15), arranged in a staircase like fashion. Their properties have the same relationship to the alkaline earth metals as the alkaline earth metals have to the alkali metals.. As you go up the group, their metallic character gets less and less. For example: boron, which is above aluminum in group IIIA (13), is not a metal but a metalloid. While these elements are ductile and malleable, they are not the same as the transition elements.These elements, unlike the transition elements, do not exhibit variable oxidation states, and their valence electrons are only present in their outer shell. All of these elements aresolid, have a relatively high density, and are opaque. They have oxidation numbers of +3, ±4, and -3. List of post-transition metals: group IIIA (13): Al Aluminum, Ga Gallium, In Indium, Tl Thallium group IVA (14): Sn Tin, Pb Lead group VA 15) : Bi Bismuth Transition metals The 38 elements in groups 3 through 12 of the periodic table are called "transition metals". As with all metals, the transition elements are both ductile and malleable, and conduct electricity and heat. The interesting thing about transition metals is that their valence electrons, or the electrons they use to combine with other elements, are present in more than one shell. This is the reason why they often exhibit several common oxidation states. three noteworthy elements in the transition metals family: iron, cobalt, and nickel -- are the only elements known to produce a magnetic field (i.e. that can be magnetized) The transition metals are the subgroups of elements intervening between groups IIA(2) and IIIA(13) in the periodic table. They are classified separately because of the filling of their d subshell orbitals. All the transition elements are metallic, but unlike the representative metals, they are likely to be hard, brittle, and have high melting points because of the relatively small size of their atoms and the existence of some covalent binding between ions. There are exceptions, as in the case of mercury (Hg), which is a liquid. They have high electrical conductivity because of delocalization of the s electrons similar to what occurs in the alkali and alkaline-earth metals. Transition metals, cont’d transition metals have a great variety of oxidation states shown in its compounds. Because of electron spin, unpaired electrons give rise to paramagnetism. Paramagnetism is likely in transition metals because of the partial filling of the d subshell and the movement associated with the orientations of electrons. Color of transition metals (and some of their ionic compounds) due to absorption of some of the frequencies of white light electronic transitions in the d subshell. The stored energy is then dissipated through heat. transition metals also have complex ionic structures because of the availability of d orbitals for participating in chemical bonding. List of transition metals: ScScandium Cr Chromium Co Cobalt Zn Zinc Nb Niobium Ru Ruthenium Ag Silver Hf Hafnium Rh Rhenium Pt Platinum Ac Actinium Ti Titanium Mn Manganese Ni Nickel Y Yttrium Mo Molybdenum Rh Rhodium Cd Cadmium Ta Tantalum Os Osmium Au Gold V Vanadium Fe Iron Cu Copper Zr Zirconium Tc Technetium Pd Palladium La Lanthanum W Tungsten Ir Iridium Hg Mercury Inner transition (rare earth) metals thirty inner transition metals (also called rare earth elements) contain 2 series of elements: lanthanide series, actinide series. All of them in group 3 of the periodic table, and the 6th and 7th periods. One element of the lanthanide series and most of the elements in the actinide series are called transuranium, which means synthetic or man-made. Lanthanide series The lanthanide series include the 15 elements from atomic numbers 57 (lanthanum) to 71 (lutetium). Their electron configuration include the 4f and 5d energy levels. Because of the closeness of those two levels, there is considerable uncertainty in some electron configuration assignments. All the lanthanides form +3 ions as their principal chemical species. It is assumed that the ions are formed by losing the 6s2 and 5d1 (or 4f is 5d is not present) electrons. They generally occur together, except for promethium which has an unstable nucleus. The richest source mineral is monazite, a complex phosphate. They are very rare to find, hence their nickname--"the rare earth elements." Since they also have very similar chemical properties, separation is very difficult, involving fractional crystallization and ion-exchange techniques. The lanthanides also generally have an incomplete 4f subshell, resulting in paramagnetism. List of lanthanides: La Lanthanum Ce Cerium Pr Praseodymium Nd Neodymium Pm Promethium Sm Samarium Eu Europium Gd Gadolinium Tb Terbium Ds Dysprosium Ho Holmium Er Erbium Tm Thulium Yb Ytterbium Lu Lutetium The Actinide series The actinide series include the 14 elements that follow actinium (atomic number 89) from atomic numbers 90 to 103. The electron configurations of the actinides are even more uncertain than the lanthanides because the closeness of the energy levels and because the nuclei are unstable to radioactive decay. Only minute amounts of some elements are obtained because of their instability. All of the actinides are unstable with respect to alpha emission. The later members tend to undergo spontaneous fission, a fact which limits the number of elements possible. The actinides also seem to show a variety of oxidation states, unlike the lanthanides. Uranium, for example, has compounds in each of the states, +3, +4, +5, and +6. Lu Lutetium Thorium (Th) Pa Protactinium U Uranium (U) Np Neptunium Pu Plutonium Am Americium Cm Curium Bk Berkelium Cf Californium Es Einsteinium Fm Fermium Md Mendelevium No Nobelium Lr Lawrencium