Acids, Bases, & Salts Worksheet: Definitions & Concepts
advertisement
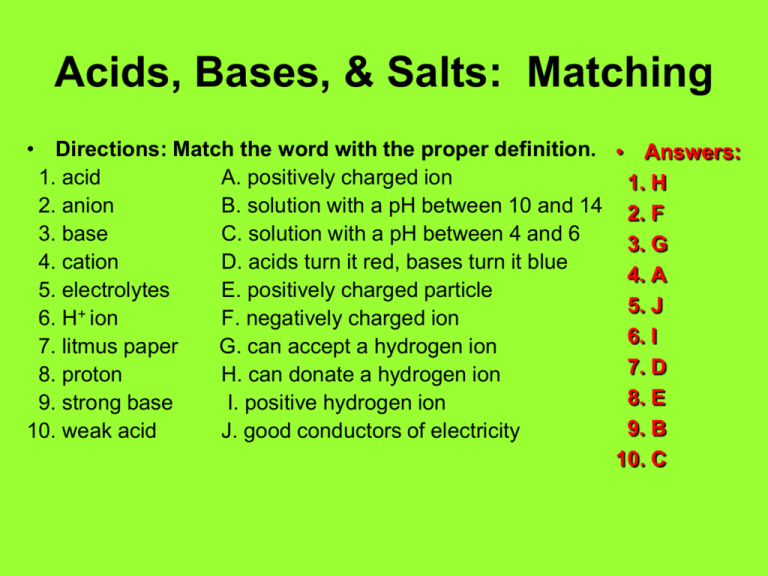
Acids, Bases, & Salts: Matching • Directions: Match the word with the proper definition. • Answers: 1. acid A. positively charged ion 1. H 2. anion B. solution with a pH between 10 and 14 2. F 3. base C. solution with a pH between 4 and 6 3. G 4. cation D. acids turn it red, bases turn it blue 4. A 5. electrolytes E. positively charged particle 5. J 6. H+ ion F. negatively charged ion 6. I 7. litmus paper G. can accept a hydrogen ion 7. D 8. proton H. can donate a hydrogen ion 8. E 9. strong base I. positive hydrogen ion 9. B 10. weak acid J. good conductors of electricity 10. C Acids, Bases, & Salts: Use the Right Word • Directions: Find the right word from the vocabulary list that completes the following sentences. 1. According to the Brønsted-Lowry definition, an ____________ is any substance that can donate a hydrogen ion. • acid 2. According to the Brønsted-Lowry definition, a ____________ is any substance that can accept a hydrogen ion. • base 3. Rainfall that is below 5.6 on the pH scale is called ____________ ____________. • acid rain Acids, Bases, & Salts: Matching • • • • • • • • • • Acid = can donate a hydrogen ion Anion = negatively charged ion Base = can accept a hydrogen ion Cation = positively charged ion Electrolytes = good conductors of electricity H+ ion = positive hydrogen ion Litmus paper = acids turn it red, bases turn it blue Proton = positively charged particle Strong base = solution with a pH between 10 & 14 Weak acid = solution with a pH between 4 & 6 Acids, Bases, & Salts: Use the Right Word 4. Substances that are good conductors of electricity are called ____________. • electrolytes 5. A special type of paper that can determine if a solution is an acid or a base is called ____________ paper. • litmus or indicator 6. The process in which acids and bases react so that the properties of both are lost to form water and a salt is called ____________. • neutralization Acids, Bases, & Salts: Use the Right Word 7. The scale that measures the concentration of H3O+ ions in solutions is called the ____________ scale. • pH 8. An ion that has fewer electrons than protons is called a ____________ ion. • positive 9. The symbol for a positive hydrogen ion is written ____________ ion. • H+ 10. The symbol for a hydroxide ion is written ____________ ion. • OH-





