Mussel Lab - Room N-60
advertisement

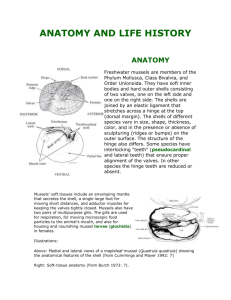
Day 1: Introduction to mussels Day 2: Mussel dissection and data collection Day 3: Deep-sea mussel data & analysis Day 1: Mussel Introduction Mussel Taxonomy: Phylum: Mollusca Class: Bivalvia Order: Mytiloida Family: Mytilidae Scientific classification helps us understand which organisms are closely related to each other. Evidence suggests that deep-sea seep mussels are evolved from intertidal mussels and vent mussels evolved from seep mussels. Intertidal Marine Mussels Basic Anatomy: • Valve (or shell), two valves: left & right • Adductor muscles, posterior & anterior • Mantle, lining the shell • Gills, two layers on each side • Siphon • Foot & Byssal threads • Digestive system (stomach) Intertidal mussels are filter feeders. Seawater in the intertidal mussels’ environment is full of plankton, algae and other photosynthetically derived food particles. The gills are also involved in respiration. Extraction of oxygen occurs primarily at the gill surface. Indigestible particles and filtered water leave the mussel through the excurrent siphon. The mussel’s gills are involved in filter feeding. The mussel draws water into its gills through an incurrent siphon. Mucous on the gills traps food particles. Cilia on the gills move the mucous and food toward the mouth. Two small labial palps sort the particles directing edible bits into the mouth. Click here to see video of the feeding process. Intertidal mussels Mytilus californianus Intertidal Mussels Review Questions • How do intertidal marine mussels feed? • Where do the organisms that intertidal marine mussel consume get their energy? Deep-sea Hydrothermal Vent Mussels Bathymodiolus thermophilus Deep-sea Cold Seep Mussels Bathymodiolus brooksi and B. childressi Deep-sea Cold Seep Mussels Bathymodiolus brooksi Think about it: In the deep-sea environment, sunlight does not penetrate to the seafloor so there is little to no photosynthetically-created food in either the seep or vent environment. How do deep-sea mussels get enough food to survive? And not just survive but thrive! Hydrothermal vent and cold seep environments review Q: What is the source of energy at deep-sea seeps and hydrothermal vents? Q: Who are the primary producers in these deep sea environments? Q: Considering how other animals in the deep–sea extreme environment get their food, where do you think deep-sea mussels get their food? Forming hypotheses: Q: Considering that intertidal mussels use their gills extensively in filter feeding, what kind of anatomical adaptations would you expect to find in a deep sea mussel? Make a prediction of what you’d expect to see in a comparison of intertidal mussels with deepsea mussels. Day 2 - The mussel lab protocol: Scientists studying deep-sea mussels have carefully examined mussel anatomy, in particular the gill tissue, and have compiled a dataset on gills from multiple dissections. To make a valid comparison we will follow the same protocol used to collect deep-sea mussel gill data. Q: Why is it important to use the same procedures in the classroom dissections as the scientists used in their dissections? What will we need? • Marine mussels • Dissecting tray • Two graduated cylinders (one small ~10ml, and one large ~250 ml) • Small, sharp knife to open mussels • Dissecting forceps • Small dissecting scissors • Plastic weighing dishes • Calipers • Water Step 1: Measure shell length Measure the length of each mussel shell, in mm. Record this on the datasheet. Step 2: Open the Mussels Open each mussel by cutting through its adductor muscles. Step 3: Examine the organs Step 4: Remove the Gill tissue (Ctenidia) Locate the gills. Note the two layers on each side. Lift the gill tissue up from the mantle and look for the line where this tissue is connected to the mantle. Using scissors, carefully cut along this line to remove the gill tissue. Be careful to get all of it, while avoiding the mantle. Gill tissue in weighing dish Place the gill tissue (all pieces, from both sides) in a plastic weighing dish, and set aside. Step 5: Next slide a knife under the mantle. Remove all visceral tissue (all soft body tissue) from the shell. Pull the mantle away from the shell, using the knife. Repeat for the other half of the shell Scrape all tissue into another plastic weighing dish. Be sure to scrape all tissue off the shell, especially the adductor muscle, which is tightly connected to the shell. Here’s what you should have: 1. A tray containing gill tissue 2. A tray containing the rest of the body tissue 3. A clean shell Volume Displacement Measurement: Step 1 Next, measure the volume of tissue. First, fill the small measuring cylinder half full of water. Record this starting volume on the datasheet. Measurement: Step 2 Place the gill into the cylinder. Make sure all the tissue is completely submerged. Measurement: Step 3 With the gill tissue submerged, note the final volume (water + gill) and record this value on the datasheet. The volume of gill can be calculated as this final volume minus the starting volume. Measurement: Step 4 Now measure the volume of body tissue using the large measuring cylinder. You may need the larger cylinder to measure the volume of rest of the mussel tissue. Follow the same volume displacement procedure used to measure gill tissue. Record your results on the datasheet. Calculations: Determine the proportion of gill volume to total mussel tissue volume. Enter your data into the class dataset and determine the class average. Predict how this will compare with deep-sea mussels. Q: Will the deep-sea mussels have the same proportion of gill tissue? Or will they be different? Day 3 - Deep-sea data comparison Retrieve the vent and seep mussel data. Calculate the average proportion of gill to total body volume for the vent and seep mussels. Read through the “Field Notes” to be sure you are familiar with how these data were obtained. Compare your class average to the seep and vent mussel averages. Are they the same or different? Q: What do you think accounts for the differences? Q: Thinking about the protocol that you followed, are there factors that might cause variables in your results? Deep-sea “Field Notes” Excerpts East Pacific Rise Cruise, May 2005 Collecting mussels from the ocean floor Although we tried to collect consistent samples, the number of mussels in each sample varied because of the uneven distribution of animals, but also because of the challenges of working in this environment. Remember, deep-sea mussels live at the bottom of the ocean, 2500 meters deep, in pitch darkness, and we need a deep-sea vehicle like Alvin to find and collect them. Often, the mussels are clumped, held together by byssal threads, and it's easy to collect a bunch. Other times, we get only a few. With each grab, the mussels are placed inside the Biobox (a heavy-duty plastic box) and brought to the surface. As soon as Alvin is on deck, the mussels are removed from the Biobox, placed in cold seawater and then stored in a walk-in refrigerator (Temperature = 2.8degrees C /37degrees F) until dissection. Dissections are typically on the same day as collection. In most cases, the biologists in Dr. Shank's lab also sample tissue from these mussels for genetic analysis. “Field Notes” continued Location of Vent Sites: Locations of vents where deepsea mussels were collected are shown on this bathymetry map. The red areas on the map are the tallest part of this section of the East Pacific Rise. Green and blue areas are deeper and colder. “Field Notes” continued Deep Sea Mussel Dissection: For each mussel, we measure shell length and then open the mussel to examine the body cavity. We dissect mussels and measure gill tissue volume and total body tissue volume. We then calculate the ratio of gill volume to total body volume. We use ratios so that we can make a fair comparison between individuals of different sizes. By the way, on nearly all of the deep-sea mussels collected, we notice a large number of byssal threads covering the shells. These threads, made of incredibly strong collagen, serve as a means for the mussels to attach themselves to the substrate. “Field Notes” continued Observations: What’s this living in here? One of the first things we notice inside many of the mussels is an abundance of eggs. So these mussels are apparently healthy and reproductive. We also see small polychaete worms in many of the mussels. This particular species of worm lives inside mussels and very little is known about it. As the mussel irrigates its own gills and filters food by moving sea water in and out of its shell, the worm likely lives off of the particles floating around inside. It may also live off of the mucus formed by the mussel, but scientists studying these worms are not entirely sure about this. Polychaete means "many bristles," which is obvious when you look closely at this worm. Putting it all together How did your class average compare to the seep and vent mussel averages. Were they the same or different? -If different, what accounts for the differences? Answer the questions on the Comparing our Data handout. These will prepare you for the FLEXE Forum. NEXT – The FLEXE Forum In March, Dr. Nicole Dubilier, from the Max Planck Institute of Marine Microbiology, will host the FLEXE Forum to discuss your findings. Dr. Dubilier is an expert on symbiosis and has studied deepsea marine organisms for many years. She will also discuss adaptations in general and the relative importance of symbiosis in deep-sea ecosystems. Stay tuned!