Plan B - Emergency Contraception
advertisement

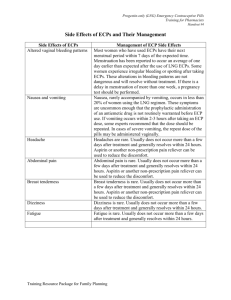
EMERGENCY CONTRACEPTION Still the Nation’s Best-Kept Secret James Trussell, PhD Office of Population Research Princeton University What if ? A condom broke or slipped off, you had sex when you did not expect to, you did not use any birth control that weekend, you missed several pills, you were forced to have sex . . . Emergency Contraceptives • • • • • • • Regular contraceptives used in a different way Prevent pregnancy after intercourse Inhibit ovulation, fertilization, or implantation Do not cause an abortion Will not interrupt an established pregnancy Are not the same as mifepristone Do not protect against STIs Definition of Pregnancy • NIH/FDA – “Pregnancy encompasses the period of time from confirmation of implantation until expulsion or extraction of the fetus.” • ACOG – “Pregnancy is the state of a female after conception and until termination of the gestation.” – “Conception is the implantation of the blastocyst. It is not synonymous with fertilization; synonym: implantation.” Options in the United States • Emergency use of oral contraceptive pills containing estrogen and progestin • Emergency use of oral contraceptive pills containing only progestin • Emergency Copper-T IUD insertion Emergency Contraceptive Pills: Combined • Ordinary birth control pills • Contain estrogen and progestin • 2 doses of 2, 4, or 5 pills, depending on brand • First dose within 120 hours after intercourse • Second dose 12 hours later • Side effects: nausea (50%) and vomiting (20%) Emergency Contraceptive Pills: Progestin-only • • • • • • • Birth control pills containing only progestin 2 doses of 1 Plan B pill or 20 Ovrette pills First dose within 120 hours after intercourse Second dose 12 hours later Both doses can be taken at the same time More effective than combined ECPs Less nausea and vomiting than combined ECPs Copper IUD Insertion • Copper-T IUD (ParaGard) • Insertion within 5 days after ovulation (but most protocols state within 5 days after unprotected intercourse) • 10 more years of highly effective contraception • Much more effective than ECPs History of EC Methods • • • • • mid-1960s: high dose estrogens early 1970s: combined OCs (Yuzpe regimen) late 1970s: copper IUD mid-1990s: levonorgestrel-only pills mid-1990s: antiprogestins Effectiveness If 1000 women have unprotected sex once in the second or third week of their cycle # of Pregnancies % Reduction No treatment 80 Combined ECPs 20 75% Progestin-Only ECPs 10 88% 1 99% IUD Insertion How Long After the Morning After? Meta-Analysis of 9 Trials (Combined) 2.5% p=.25 Pregnancy Rate 2.0% 1.5% 1.0% 0.5% 0.0% Day 1 Day 2 Day 3 How Long After the Morning After? WHO (Combined and LNg) 5.0% Pregnancy Rate 4.0% p<.01 3.0% 2.0% 1.0% 0.0% 0-12 13-24 25-36 37-48 49-60 61-72 How Long After the Morning After? Quebec (Combined) 1.2% Pregnancy Rate 1.0% p=.75 87 92 Days 1-3 Days 4-5 0.8% 0.6% 0.4% 0.2% 0.0% How Long After the Morning After? Population Council (Combined) Pregnancy Rate 4.0% 3.5% 111 3.0% 2.5% 2.0% 1.5% 675 589 104 1.0% 0.5% 0.0% Days 1-3 p=.52 and .99 Days 4-5 Typical Perfect How Long After the Morning After? Latest WHO Trial (LNg) 2.5% p=.16 Pregnancy Rate 2.0% 314 1.5% 1.0% 2381 0.5% 0.0% Days 1-3 Days 4-5 How Long After the Morning After? Chinese Trial (LNg) 3.0% p=.26 Pregnancy Rate 2.5% 139 2.0% 1.5% 1932 1.0% 0.5% 0.0% Days 1-3 Days 4-5 The Setting • 3.1 million unintended pregnancies each year in the United States: half (49%) of all pregnancies • Half (48%) of women aged 15-44 have ever had an unintended pregnancy • Emergency contraception has the potential to reduce unintended pregnancy significantly • Emergency contraception is highly costeffective Potential Impact Reduce unintended pregnancies by half 1.5 million fewer Reduce abortions needed by half 0.7 million fewer Reduce pregnancies after rape by 88% 22 thousand fewer Potential Unrealized • 75 million cycles per year in which unprotected intercourse occurs among women at risk of an unintended pregnancy • Only 6% of women have ever used ECPs The Problem • Companies did not market pills or IUDs for emergency contraception in the United States • Clinicians do not routinely counsel women (or men) about emergency contraception • Women (and men) do not know about emergency contraception • Pharmacies do not routinely carry ECPs The Solution • Market EC • Change provider practices • Enhance availability – Establish call-in prescription services – Enhance pharmacy access – Change from Rx to OTC • Educate women (and men) MARKETING Preven Gynétics 1998-2004 The Value of a Dedicated Product Ovral Preven Alesse Plan B WCC 1999 The Value of a Dedicated Product Plan B Ovrette EC in Europe PC4 Schering Postinor-2 Gedeon Richter NorLevo HRA Pharma • OTC in Norway (2000), Sweden (2001), Netherlands (2004), India (2005), US (2006) • Dispensed by school nurses in every junior and senior high school in France • And dispensed at no cost to minors by pharmacists PROVIDER PRACTICE Providing EC is Now the MedicoLegal Standard of Care • ACOG Practice Pattern on ECPs (12/96) established the professional standard of care • FDA notice in Federal Register on ECPs (2/97) declared 6 (now 20) brands of regular OCs to be safe and effective for use for emergency contraception • FDA explicitly approved Preven and Plan B as dedicated products, but FDA still recognizes 19 brands of regular combined OCs to be safe and effective for use for EC Provider Practice: Good News 100% OB/GYNs 80% 80% FPPs 60% 40% 36% 20% 0% Prescribed ECPs Last Year Provider Practice: Bad News 50% 40% 39% OB/GYNs 30% 20% 25% 17% FPPs 14% 10% 0% Prescribed More Than Five Times Among Those Who Prescribed Rountinely Discuss EC The Clinical Bottleneck • Clinicians overwhelmingly think ECPs are safe and effective, and the majority have prescribed in the last year • And clinicians are waiting for women to ask for EC • But women do not know to ask – while 68% of women have heard of ECPs/MAPs – only 6% of women have ever used ECPs INCREASING ACCESS EC Hotline and Website • Emergency Contraception Hotline – 1-888-NOT-2-LATE – 630k calls since 1996 • Emergency Contraception Website – http://not-2-late.com – 3.2m visits since 1994 Providers on the Hotline and Website State Websites: Prescriptions Called In • • • • • • • • • • • Georgia: www.ecconnection.org Illinois: www.plannedparenthoodchicago.com Indiana: www.ppin.org/ecaccess/ecinfo.html Maine: www.ppnne.org Massachusetts: www.pplm.org North Carolina: http://www.pphsinc.org/ec/ Oregon: www.ppcw.org South Carolina: http://www.pphsinc.org/ec/ Vermont: www.ppnne.org Washington: www.ppcw.org West Virginia: http://www.pphsinc.org/ec/ Statewide Hotlines: Prescriptions Called In • • • • • • • • • • • • • • Connecticut: 800-230-PLAN Georgia: 877-ECPills Illinois : 866-222-EC4U or 217-544-2744 Maryland: 877-99-GO-4-EC Massachusetts: 800-682-9218, 642-5665, 539-2378 Michigan: 734-973-0710 Minnesota: 612-625-4607 Montana: 800-584-9911 New Mexico: 505-272-9304 New York: 585-271-9055 North Carolina: 866-942-7762 South Carolina: 800-230-PLAN West Virginia: 800-230-PLAN Wisconsin: 877-975-9858 States with Call-in Prescriptions 39% of women aged 15-44 Emergency Contraception BTC ECPs are available directly from pharmacists without having first to get a prescription in: • • • • • • • • Alaska California Hawaii Maine Massachusetts Montana New Hampshire New Mexico • • • • • • • • Vermont Washington Canada France United Kingdom Australia South Africa 33 other countries+5 OTC Pharmacists Providing ECPs Plan B OTC • Application to switch from Rx to OTC submitted April 20, 2003. FDA decision due by February 20, 2004 • FDA convened advisory committee on December 16, 2003. Committee voted 23-4 in favor • FDA announced on February 13, 2004 that it would delay the decision by up to 90 days • FDA rejected application on May 6, 2004, citing lack of data on females <16 Evidence-Based Decision? • In 10 studies, women were randomly assigned to get ECPs in advance or in the regular way • 1 study among teens, 1 among mothers 1320, 2 among women 15-24 • Not one study found an increase in risk taking, or a reduction in condom use, or abandonment of regular contraceptive use • FDA has never previously required data on persons <16 GAO Report: Decision Very Unusual • Report requested by Congress in June 2004 and released in October 2005 • Report concludes that the decision was highly unusual, was made with atypical involvement from top agency officials, and may well have been made months before it was formally announced Plan B OTC • Barr Laboratories submitted amended application on July 22, 2004, to make Plan B an Rx drug for females <16 and OTC otherwise • FDA had until January 21, 2005 to respond • On July 15, 2005, HHS Secretary Leavitt promised that FDA would act on Barr's application by September 1 to ensure a vote on Senate confirmation of Lester Crawford as FDA Commissioner Plan B OTC • On August 26, 2005, FDA announced that Plan B was safe for OTC use by women ≥17 • But FDA announced an indefinite delay in reaching a decision, citing three concerns: – Can Plan B be both Rx and OTC depending on age? – Can Rx and OTC versions of the same drug be marketed in the same package – Can an age restriction be enforced? • 60-day public comment period on first two Plan B OTC • Three days later, Susan Wood resigned her position as the Assistant Commissioner for Women's Health and Director of the FDA Office of Women's Health, stating that: “I have spent the last 15 years working to ensure that science informs good health policy decisions. I can no longer serve as staff when scientific and clinical evidence, fully evaluated and recommended for approval by the professional staff here, has been overruled.” Plan B OTC • On July 31, 2006, the day before his confirmation hearing, acting FDA Commissioner Andrew von Eschenbach publicly invited Barr Labs to resubmit its application by changing the OTC age restriction to 18 and over. • On August 18, 2006, Barr Labs resubmitted its application. • On August 24, 2006, the FDA approved. Department of Justice • National Protocol for Sexual Assault, 9/04 • Extensive 4-page discussion of STI counseling, testing, and prophylaxis • Short ½-page discussion of pregnancy risk evaluation and care • No mention of emergency contraception Department of Defense • Basic Core Formulary (BCF) is a list of medications that are required to be on all Military Treatment Facility formularies • The Pharmacy & Therapeutics Committee (P&TC) is responsible for changes to the BCF • Plan B added to the BCF on April 3 2002 but removed on May 8 after intervention by the Assistant Secretary of Defense for Health Affairs Are ECPs Effective? • Eight of the ten studies conducted to test whether easy assess to ECPs increased risk taking also measured pregnancies • In none of the eight did advance provision of ECPs reduce pregnancy rates • Only three studies powered to detect a decrease in pregnancy rates Why No Reduction in Pregnancies? • In San Francisco almost half of the women in the advance provision group who had unprotected intercourse did not use ECPs • In China, 30 of the 38 pregnancies in the advance provision group occurred to women who did not use ECPs in that cycle • In Nevada/NC, 57 of the 74 pregnancies in the advance provision group occurred to women who did not use ECPs in that cycle • Lesson: ECPs are not used frequently enough! Source: Raine et al. 2005; Hu et al. 2005; Raymond et al. 2006 Advance Provision of ECPs Did Not Reduce Abortions Rates in Lothian • Community intervention study in Scotland • About 1 in 5 women aged 16-29 got ECPs in advance to take home • About half of these used ECPs at least once • No effect on abortion rates was observed • Women most at risk probably did not get ECPs • 78% of women with advance supplies who got pregnant did not use ECPs. Source: Glasier et al. 2004 Excellent Evidence that Plan B Works • Two trials in which women were randomly assigned to Plan B or Yuzpe regimen. • Pregnancy rate in Plan B arm was 51% of the rate in the Yuzpe arm. • Plan B is 49% effective if Yuzpe regimen is completely ineffective. • If, for example, Yuzpe regimen is 60% effective, then Plan B is 79% effective. Source: Raymond et al. 2004 Lesson Learned • ECPs are not used nearly frequently enough! • Women underestimate their risk of pregnancy • More education is needed • OTC switch is necessary―but not sufficient―for solving this problem EDUCATION Public Education Campaign Messages • There is something that can be done after unprotected sex to prevent pregnancy • To find out more, call 1-888-NOT-2-LATE • There is a 72-hour time limit