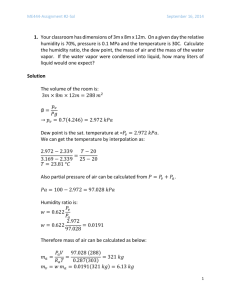
File - Chemistry @ BCS
advertisement

Section 5 Exam Questions and Answers
2002 Question 8
The following hydrocarbons can all be used as fuels.
methane (CH4)
butane (C4H10)
2,2,4-trimethylpentane (C8H18)
(a) Butane is a major component of LPG. What do the letters LPG stand for?
(5)
Draw two structural isomers of butane. (6)
(b) Methane is a major component of natural gas. Why are mercaptans often added to natural gas?
What environmental change or effect is associated with the release of methane to the atmosphere?
Apart from leaking gas pipes, name a major source from which methane is released to the atmosphere. (9)
(c) What structural feature of 2,2,4-trimethylpentane results in it having a high octane rating?
Give one other structural feature which increases the octane number of a hydrocarbon.
(6)
(d) Define heat of combustion of a compound.
(6)
(e) The combustion of butane is described by the following balanced equation.
2C4H10(g) +13O2(g) → 8CO2(g) + 10H2O(l)
Calculate the heat of combustion of butane given that the heats of formation of butane, carbon dioxide and water are –
125, –394 and –286 kJ mol-1, respectively.
(18)
2002
Question 8
(a)
LPG: liquefied (liquid) petroleum gas
(5)
(6)
[Accept sticks without Hs put in] (3)
[Accept sticks without Hs put in] (3)
WHY: to give an odour (smell) / to detect leaks (3)
WHAT: greenhouse effect / global warming / reducing ozone damage (3)
SOURCE: fossil fuels / oil / coal / marshes (bogs, paddy fields) / animals (cows, sheep, etc.)
/dumps / slurry / anaerobic decay / compost (3)
FEATURE: branching / short chain
OTHER: ring / branching (unless used above) / aromatic / short chain (if not used above)
DEFINE: heat when 1 mole (3) is burned completely / burned in excess oxygen
CALCUL: – 2881 kJ mol–1 (18)
ΔH = ΔHformation(products) - ΔHformation(reactants)
ΔH = [4 (3) ( – 394) (3)+ 5 (3) (– 286) (3) – (– 125) (3)] = – 2881 kJ mol–1 (3)
OR
[ – 1576 (6) – 1430 (6) + 125 (3)] = – 2881 kJ mol–1 (3)
(b)
(c)
(d)
2003
(3)
(3)
(3)
(18)
Question 10. (a)
Define heat of combustion.
(7)
Propane may be used in gas cylinders for cooking appliances. Propane burns according to the equation
C3H8 + 5O2 3CO2 + 4H2O
(i) The heats of formation of propane, carbon dioxide and water are –104, –394 and –286 kJ mol–1 respectively. Calculate
the heat of combustion of propane.
(12)
(ii) If 500 kJ of energy are needed to boil a kettle of water what mass of propane gas must be burned to generate this
amount of heat? Express your answer to the nearest gram.
(6)
2003
Question 10. (a)
(a)
(i)
(ii)
Define: heat when 1 mole (4) is burned completely / burned in excess oxygen
Calc: ΔH = – 2222 kJ mol–1
Σ ΔH formation(products) – Σ ΔH formation(reactants) = ΔH
3 × – 394 or – 1182 (3) 4 × – 286 or – 1144 (3) – (–104) /or + 104 / 104 (3) = – 2222 kJ mol–1 (3)
10 g
1 × 500 = 0.225 mol (3) × 44 = 10 (3) OR 44 × 500 (3) = 10 (3)
Note: penalty (– 1) if answer
2222
2222
not rounded off to 10.
(3)
(12)
(6)
2004
Question 6
(a) Define (i) heat of formation of a substance, (ii) octane number of a fuel.
(11)
(b) The combustion of methane is described by the following balanced equation.
CH4(g) + 2O2(g) CO2(g) + 2H2O(l) ΔH = − 890.4 kJ mol-1
The standard heats of formation of carbon dioxide and water are −394 and −286 kJ mol-1 respectively.
Calculate the heat of formation of methane. (12)
(c) Methane is an excellent fuel. Give two properties of methane which account for its usefulness as a fuel.
Natural gas is a rich source of methane. Why are mercaptans often added to natural gas?
(9)
(d) Methane is often found in gas fields which occur in association with crude oil deposits. Crude oil is fractionated in
order to obtain more useful products. Outline clearly how the fractionation process is carried out.
(12)
(e) Identify two structural features of a hydrocarbon fuel which affect its octane number.
(6)
2004
(a)
(b)
(c)
(d)
(e)
Question 6
(i)
(ii)
heat (energy) when 1 mole //of compound formed from its elements
measure of tendency to auto-ignite (knock) or number representing ability of fuel to resist
auto-igniting (knocking)
CALC.: – 75.6 kJ mol–1
ΔH(reaction) = Σ ΔHf(products) – Σ ΔHf(reactants)
– 890.4 (3) = [ – 394 (3) – 572 (3)] – [ΔHf(methane) + 0]
ΔHf(methane) = – 394 – 572 + 890.4 = – 75.6 kJ mol–1 (3)
[Allow 3 marks only for +75.6 kJ mol–
1 ]
PROPS: high kilogram cal. value (high heat of combustion) / clean (non- polluting) / non-toxic /
relatively cheap/ can be piped Do not accept ‘easily distributed in tanks’
ANY TWO: (2 x 3)]
WHY: to give an odour (smell) / to detect leaks / to make safe
OUTLINE: crude oil heated / crude added continuously at bottom /
vapour passes up through column / fractionating tower with trays
fractions condense at different levels depending on their boiling points / high b.p. fractions at
bottom / low higher up /heavier at bottom / lighter at top / or named exemplar to indicate this
[The first three points can be got from a diagram; the last point must be specified in words or clearly
written on the diagram.]
ANY THREE: (6 + 2 x 3)
IDENTIFY: chain length / branching / cyclic / aromatics
ANY TWO: (2 x 3)
(3+2)
(6)
(12)
(6)
(3)
(12)
(6)
2005 Question 6
(a) The octane number of a fuel is described as a measure of the tendency of the fuel to cause knocking, or as a measure of
the tendency of the fuel to resist auto-ignition. This number is found by comparing the combustion of the fuel with the
combustion of a mixture of two reference hydrocarbons using the same standard engine.
(i) Name both of the reference hydrocarbons present in the mixture used when measuring octane number by this
comparison method.
(8)
(ii) State two structural features of a hydrocarbon molecule which contribute to it having a high octane number. (6)
(iii) Lead compounds were used in the past to increase the octane number of fuels. Why are lead compounds unsuitable as
additives for petrol used in modern cars?
(3)
(iv) Identify one additive or type of additive, other than a compound of lead, used to increase the octane number
of fuels.
(3)
(b) There are three structural isomers of the hydrocarbon of formula C5H12. In the case of each of these isomers, draw the
structure of the molecule and give its systematic IUPAC name.
(18)
(c) The combustion of liquid benzene is described by the following equation:
2C6H6(l) + 15O2(g) 12 CO2(g) + 6H2O(l)
Given that the heats of formation of carbon dioxide gas, liquid water and liquid benzene are –394,–286 and
49 kJ mol-1 respectively, calculate the heat of combustion of liquid benzene.
(12)
2005
(a)
Question 6
(i)
(ii)
(iii)
(iv)
(b)
(c)
NAME: 2,2,4-trimethylpentane (isooctane) // heptane (n-heptane) (2 x 4)
STATE: short chain length / branching / ring (cyclic) / aromatic
ANY TWO: (2 x3)
WHY: catalyst poison / destroys catalytic converter
IDENTIFY: oxygenate or methanol or ethanol / methyl-t-butyl ether (MTBE)
CH3CH2CH2CH2CH3 / CH3(CH2) 3CH3 (3) pentane (3)
(CH3) 2CHCH2CH3 / CH3CH2CH(CH3) 2 (3) 2-methylbutane (3)
(CH3) 4C / (CH3) 3CCH3 / (CH3) 2C(CH3) 2 (3) 2,2-dimethylpropane (3)
[Matching of names and formulae are required. In expanded structures, bonds without Hs are
acceptable. Numbers are not required for the methyl branches but, if incorrect numbers are offered
(e.g. 1,2-dimethylpropane), then no marks should be awarded.]
ΔH = Σ ΔH
– Σ ΔH
f(products)
f(reactants)
(8)
(6)
(3)
(3)
(6)
(6)
(6)
(12)
ΔH = 12 x –394 or – 4728 (3) + 6 x –286 or –1716 (3) – {2 x 49 or 98 (3) + 0}
= – 6542 => ΔHc = – 3271 (3)
[Allow 3 marks only for + 3271 kJ mo-1]
2006
Question 6
(a) The table shows the octane numbers of four hydrocarbons.
(i) What is meant by the octane number of a fuel?
(8)
(ii) Hexane has the lowest octane number of the four compounds listed.
What structural feature of the molecule contributes to this?
(3)
(iii) In the case of each of the other three compounds, identify the
structural feature of its molecules which contributes to it having a high
octane number.
(9)
(iv) Name the process carried out in an oil refinery that converts hexane
to compounds such as cyclohexane and benzene.
Why is the use of benzene in petrol strictly controlled?
(6)
(b) (i) Give two reasons why oxygenates such as MTBE are added to petrol. (ii) Give two reasons why the addition of
lead to petrol has been discontinued.
(12)
(c) The combustion of cyclohexane may be described by the following balanced equation:
C6H12(l) + 9O2(g) 6CO2(g) + 6H2O(l)
Given that the heats of formation of cyclohexane, carbon dioxide and water are –156, –394 and –286 kJ mol–1,
respectively, calculate the heat of combustion of cyclohexane. (12)
2006
(a)
Question 6
(i)
(ii)
(iii)
(iv)
(b)
(i)
(ii)
(c)
WHAT: measure of tendency to auto-ignite (knock) or number representing ability of fuel to
resist auto-igniting (knocking)
WHAT: straight chain / unbranched
IDENTIFY: cyclohexane: ring / cyclic
benzene: aromatic [Accept ring / cyclic]
2,2,4-trimethylpentane: branched
PROCESS: dehydrocyclisation
WHY: benzene is carcinogenic or benzene is toxic (poisonous)
GIVE: high octane rating (number) / reduces knocking
produce clean products / reduce pollution / more complete oxidation / less carbon monoxide
produced /do not poison catalyst in converter (2 × 3)
GIVE: it poisons (destroys) the catalyst in catalytic converter //
lead emission presents a health hazard / toxic (poisonous) to living things (2 × 3)
[Do not accept ‘lead is a pollutant’ or ‘it damages the environment’]
–1
– 3924 kJ mol (12)
ΔH = Σ ΔH
– Σ ΔH
f(products)
(8)
(3)
(3)
(3)
(3)
(3)
(3)
(6)
(6)
(12)
f(reactants)
ΔH = 6 × –394 or –2364 (3) + 6 × –286 or –1716 (3) – {1 × –156 or –156 (3) + 0}
=> ΔHc = – 3924 (3)
2007
Question 6
Useful hydrocarbons are obtained by the fractional distillation of crude oil, which itself has little or no direct use.
Hydrocarbons are excellent fuels.
(a) In which fraction of crude oil do pentane and its isomers occur?
(5)
Give the systematic (IUPAC) name of each of the structural isomers of pentane shown below.
(9)
Which of these isomers would you predict to have the lowest octane number? Justify your choice in terms of the structural
features of the molecules.
(9)
Write a balanced equation for the combustion of pentane (C5H12) in excess oxygen.
(6)
(b) Naphtha and gas oil are two of the hydrocarbon fractions obtained from the fractional distillation of crude oil. How do
the molecules of the naphtha fraction differ from the molecules of the gas oil fraction?
(3)
Explain with the aid of a labelled diagram how naphtha (b.p. approximately 100 ºC) is separated from gas oil
(b.p. approximately 300 ºC) in the fractional distillation of crude oil.
(9)
Bitumen is a residue fraction obtained from crude oil. Give one use for bitumen.
(3)
(c) What is catalytic cracking? What is its economic importance in oil refining?
(6)
2007
(a)
(b)
(c)
Question 6
WHICH: light gasoline (petrol) [Accept “petroleum”]
[Allow “second highest fraction” or “from C5 to C10(C11)”]
GIVE: pentane // 2-methylbutane // 2,2-dimethylpropane (3 x 3)
[Numbers are not required as the structures are unambiguous but no marks should be awarded if
incorrect numbers are used]
WHICH: pentane or the one on the left
JUSTIFY: pentane is a straight (unbranched) chain molecule
[“longest chain” or “not highly branched” are not acceptable.]
WRITE: C5H12 + 8O2 → 5CO2 + 6H2O
FORMULAS: (3) BALANCING: (3)
HOW: naphtha (they) have shorter (smaller, less carbon atoms, smaller mass, lighter) chains /
gas oil have longer (bigger, more carbon atoms, bigger mass, heavier) chains
EXPL: diagram with one correct label (3)
[layers or outlets must be shown; outlets may be shown
by tubes (pipes), holes, gaps, lines, arrows (→)]
heat (boil) or pass vapour up tower (column) or temperature
gradient shown (3)
naphtha condenses higher up or gas oil comes off lower down (3)
GIVE: road surfacing / roofing / waterproofing (3)
WHAT: splitting (breaking) of long chain molecules by heat and catalyst(s)
[Accept “hydrocarbons” for “molecules”]
ECON: more demand for products / products used as feedstock for chemical industry / gives
higher octane numbers
(5)
(9)
(3)
(6)
(6)
(3)
(12)
(3)
(3)
2008 Question 6
(a) The hydrocarbon molecules in petrol typically contain carbon chains with between five and ten carbon atoms. The
most widely used petrol in Ireland has an octane number of 95.
(i) What is meant by the octane number of a fuel?
(5)
(ii) The two hydrocarbons used as references when establishing the octane number of a fuel are
heptane and 2,2,4-trimethylpentane. Draw the structure of each of these molecules.
(6)
(iii) Crude oil is separated into a number of fractions in oil refining. Name the two fractions which
contain molecules with the carbon chain lengths needed for petrol.
(6)
(iv) Dehydrocyclisation is one of the processes used to increase the octane numbers of hydrocarbons.
What two changes to the hydrocarbon molecules occur during this process?
(6)
(v) Ethanol is an example of an oxygenate. Give another example of an oxygenate.
Give two reasons why oxygenates are added to petrol.
(9)
(b) Write a balanced chemical equation for the combustion of ethanol, C2H5OH. Given that the heats of formation of
ethanol, carbon dioxide and water are –278, –394 and –286 kJ mol–1, respectively, calculate the heat of combustion of
ethanol.
(18)
2008
(a)
Question 6
(i)
(ii)
(iii)
(iv)
(v)
(b)
(c)
2008
WHAT: measure of tendency to auto-ignite (knock,) / number representing ability to resist autoignition (knocking, etc.) /
DRAW: heptane: CH3CH2CH2CH2CH2CH2CH3 / CH3(CH2)5CH3 /
(5)
NAME: light gasoline / petroleum (3) naphtha (3)
WHAT: removal (loss) of hydrogen [Accept “hydrogen produced”.] (3)
ring (aromatic, cyclic) formation (3)
EXAMPLE: methanol or methyl-t-butyl ether (MTBE) [Accept correct formula] (3)
GIVE: raise octane number / decrease knocking (3)
less pollution (CO) produced or more environmentally friendly or alternatives for lead (3)
[Accept “less harmful gases”, “less harmful to environment”, but not “less harmful”.]
WRITE: C2H5OH + 3O2 → 2CO2 + 3H2O
FORMULAS: (3) BALANCING (3)
CALC: – 1368 kJ mol–1
ΔH = Σ ΔHf(products) – Σ ΔHf(reactants)
ΔH = 2 × – 394 or – 788 (3) + 3 × – 286 or – 858 (3) – {–278 (3) + 0}
ΔH = – 1368 (3)
(6)
(6)
(6)
(9)
(6)
(12)
Question 11 (a)
Alcohols can be obtained by the reduction of aldehydes and ketones using hydrogen and a suitable catalyst.
(i) Name a suitable catalyst for these reduction reactions.
(4)
(ii) Name the alcohol produced when propanal (C2H5CHO) is reduced.
(3)
(iii) Draw the structure of the alcohol produced when propanone (CH3COCH3) is reduced. To which class (primary,
secondary or tertiary) of alcohols does it belong?
(6)
(iv) Which of the two compounds, propanal or propanone, would be oxidised by warm Fehling’s solution?
Give the name and structure of the organic product of the oxidation reaction.
(9)
(v) Give one common use for propanone.
(3)
2008
(b)
Question 11
(i)
(ii)
(iv)
MASS: 1144 g (6)
143 x 8 = 1144 (6)
MOLES: 26 mol
1144 ÷ 44 (3) = 26 (3)
To be accepted as a slip, some work must be shown in these calculations.
VOLUME: 624 litres
26 x 24 (3) = 624 (3)
(6)
SUV: 528 litres
(6)
[Note: subtraction step (3); other step(s) (4)]
[In part (iii), using 22.4 for 24 loses the 3 (4) marks for that step but the candidate is penalised once
only. The same applies to the use of PV = nRT except in cases where the correct answer is obtained.]
(6)
(6)
2009
Question 6
(a) Define (i) hydrocarbons, (ii) structural isomers.
(8)
(b) Give a use for the kerosene fraction obtained when crude oil is fractionated.
Explain why some of the kerosene produced in oil refining is subjected to catalytic cracking.
(9)
(c) Straight chain molecules of C13H28 occur in the kerosene fraction. Upon cracking a molecule of
C13H28, a C2H4 molecule, a C4H8 molecule and an unbranched alkane molecule are obtained.
Identify this unbranched alkane molecule and state its octane number.
Draw structures for three of the isomers of C4H8.
(15)
(d) Name two other processes carried out in oil refineries to modify hydrocarbon structures.
(6)
(e) The combustion of one of the C4H8 isomers is described by the following balanced equation.
C4H8 + 6O2 4CO2 + 4H2O
ΔH = –2710 kJ mol–1
The standard heats of formation of water and carbon dioxide are –286 and –394 kJ mol–1, respectively.
Calculate the heat of formation of this C4H8 isomer.
(12)
2009
(a)
Question 6
(i)
(ii)
(b)
(c)
(d)
(e)
compounds of* carbon (C) and hydrogen (H) only (4)
[*if “containing” is used, then “only” has to accompany it]
(ii) compounds with same molecular* formula but different structural formulas ([*not ‘chemical’] (4)
GIVE: home heating / used as paraffin oil / jet (aviation, aircraft) fuel / bus fuel / rocket fuel /
storing reactive elements (metals e.g. alkali metals; non-metals e.g. white phosphorus) /
solvent / lubricant / lighting /(paraffin lamps) / cooking / produce petrol (gasoline) / camping
stoves [Note: medicinal liquid paraffin and paraffin wax (candles) are not kerosene derivatives.]
EXPLAIN: greater demand for shorter chains (smaller molecules, kerosene chains too long) //
useful products // lighter fractions // increased octane number // production of raw materials
(alkenes – or named alkene, monomers) for making polymers (plastics, petrochemicals) (6 + 3)
[The first correct point from GIVE or EXPLAIN gets (6)].
IDENTIFY: heptane or C7H16 or CH3(CH2)5CH3 (3)
STATE: octane number: zero) [IDENTIFY & STATE are linked] (3)
NAME: isomerisation // reforming (dehydrocyclisation) (2 x 3)
CALCULATE: – 10 kJ mol–1 (12)
C4H8 + 6O2 = 4CO2 + 4H2O
ΔH = – 2710 kJ mol–1
ΔH(reaction) = ΔHf (products) – ΔHf (reactants)
– 2710 = 4 x –394 or –1576 + 4 x –286 or –1144 – ΔHf(compound) *
ΔHf(compound) = 4 x –394 or –1576 (3) + 4 x –286 or–1144 (3) + 2710** (3)
= – 10 kJ mol–1 (3)
*Give 3 for – 2710 only if the full equation in this line is given correctly
(8)
(9)
(15)
(6)
(12)
6. (a) Give the systematic (IUPAC) names for the three hydrocarbon compounds X, Y and Z. (8)
(b) Compounds X and Z are obtained from the same fraction in the oil refining process. Name the fraction in which X and
Z occur. What two properties of the compounds are responsible for them being found in the same fraction? (9)
(c) What is meant by auto-ignition in petrol engines? Compound Y has an octane number of 83 and therefore has the same
octane rating as an 83:17 mixture of two reference hydrocarbons.
Name the reference hydrocarbon that is the major component of the 83:17 reference mixture. (6)
(d) Define heat of combustion. Outline how the heat of combustion of X could be measured using a bomb calorimeter.
(15)
(e) In order to increase its octane rating, compound X is converted to compound Z in oil refineries by the following
reforming (dehydrocyclisation) process:
C7H16 (l) C7H8 (l) + 4H2 (g)
Calculate the heat change for this reaction given that the heats of formation of C7H16 (l), and
C7H8 (l) are –224.2 and 12.4 kJ mol–1, respectively.
State one important industrial use for the hydrogen produced in this reaction. (12)
QUESTION 6
(a) GIVE X: Heptane //Y: Cyclohexane //Z: Methylbenzene [(2 × 3) + 2]
(b) NAME: Naphtha / light gasoline / petrol (3) WHAT: Similar carbon number (molecular mass) // similar boiling
points (2 × 3)
(c) WHAT: Tendency to premature ignition (explosion) / tendency towards “knocking” /ignition before spark / ignition
before piston reaches top of its ascent (3)
NAME: 2,2,4-trimethylpentane / iso-octane [Italics and hyphen not required] (3)
(d) DEFINE: Energy released (heat change) when one mole of a substance //
is burned (reacts) completely in oxygen / is burned in excess oxygen (2 × 3)
OUTLINE: Place known amount (mass) of substance in a bomb calorimeter / sample in crucible //
Pressurise with oxygen / burn in excess oxygen / burn completely in oxygen /
oxygen inlet labelled in diagram //
Place bomb in known quantity water //
Ignite electrically / ignition coil (wire) labelled in diagram //
Find heat produced using the rise in temperature and the heat capacity of the
system / calculate heat produced using equation Q = mcΔT (Q = CΔT) //
From this calculate the heat produced for one mole of the substance (ANY THREE: 3 × 3)
(e) CALC.: 236.6 kJ mol–1 {– 236.6 gets (3) only} (9)
ΣÄHf(products) – ΣÄHf(reactants) = ÄH(reaction)
12.4 (3) – (– 224.2) (3) = 236.6 (3)
OR 12.4 (3) + 224.2 (3) = 236.6 (3)
STATE: Hydrogenation
(hardening) of vegetable oils (fats) (making margarine) / fuel /preparation of hydrochloric
acid (ammonia, methanol) (3)
2011 Q 6
(a) What is the nature of the chemicals that make up the bulk of crude oil? (5)
(b) Unprocessed crude oil, obtained by drilling on land or at sea, is not generally useful. Describe
with the aid of a labelled diagram how crude oil is separated into useful substances in an oil refinery.
Give the major use for the light gasoline and naphtha fractions of crude oil. (15)
(c) What is catalytic cracking? Why is it carried out in oil refining? (9)
(d) Hydrogen gas can be obtained industrially by the reaction between natural gas and water in the form
of steam (steam reforming).
(i) Describe another method by which large quantities of hydrogen can be obtained from water.
(ii) State one disadvantage of using hydrogen as a fuel. (9)
(e) Steam reforming takes place according to the following balanced equation:
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
Calculate the heat of this steam reforming reaction given that the heats of formation of methane,
steam and carbon monoxide are –74.6, –242 and –111 kJ mol–1 respectively. (12)
(a) NAT:
(b)
hydrocarbons (cpds of C and H)
[Accept ‘alkanes (paraffins)’]
DESC: hot crude oil introduced at base of fractionating
column / heated at bottom of column / heated below trays //
(5)
vapour moves up through a series of trays (levels) //
fractions come off through outlets /
fractions come off at these levels (trays) //
depending on their boiling points /
higher bp (heavier) fractions come of at lower levels /
lower bp (lighter) fractions come off at higher levels /
temperature gradient shown / highest T at bottom (lowest T at top)
(4 × 3)
[In the absence of a diagram with at least two labels deduct (3) marks.]
USE: petrol / motor fuel
(c) WHAT: splitting (breaking) of long chain molecules (hydrocarbons) //
by the action of heat and catalyst(s) / to give short chains (small molecules)
WHY:
(d) (i)
(ii)
(e)
gives useful products (more demand for products) / products needed for petrol /
products used as feedstock for chemical industry (source
of alkenes, source of ethene) / gives products with higher octane numbers
electrolysis
difficult to store / difficult to transport / not easily liquefied / tanks have to
withstand high pressures / low density (light) / explosive
ΔH = 205.6 kJ mol–1
Equations not required
ΔH(reaction)
=
ΣΔHf(products) – ΣΔHf(reactants)
=
– 111 (3) – {– 74.6 (3) – 242 (3)}
or
=
– 111 (3) + 74.6 (3) + 242 (3)
=
205.6 kJ (3)
CALC:
(3)
(2 × 3)
(3)
(6)
(3)
(12)