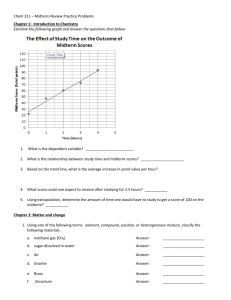
Atomic Structure and Theories
advertisement
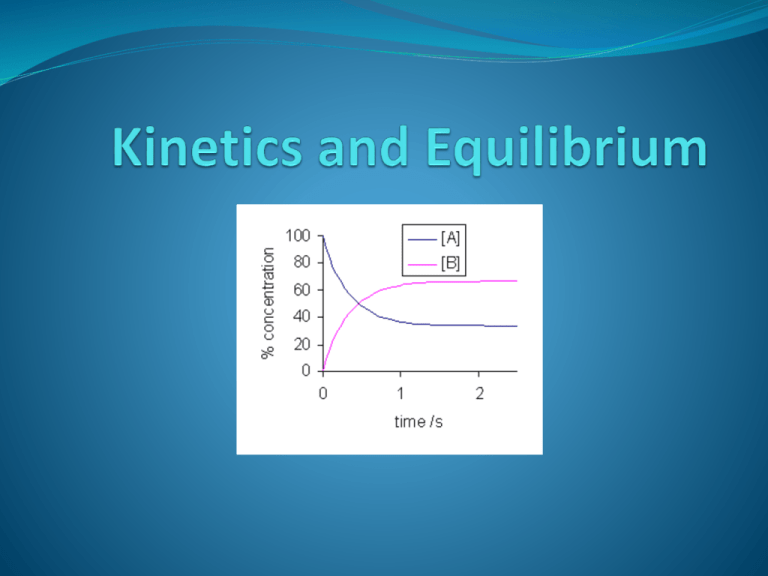
Kinetics The study of the mechanisms of a reaction and the rates of reaction. Factors that effect Rate of Reaction (R of R) Collision Theory Anything that will increase the number of and frequency of the collisions More effective collisions Factors that Effect Rates of Reactions (T,A,P,S,N,C) Temperature -(Average Kinetic Energy=motion) Inc. Temperature = Increase R of R Amount Increase Amount (concentration) = Increase R of R Pressure (g only) - Inc Pressure = increase R of R Surface Area – Inc. S.A. = Inc. R of R Nature of Reactants (Ionics > Covalents) Due to their reactivity and the number of bonds needed to break Catalyst – anything that is added that will increase the R of R If present will increase the R of R How? By providing an alternative pathway for the Reaction by effecting 3 things 1. Ea of the Forward catalyzed Reaction 2. Ea of the Reverse Catlayzed Reaction 3. PE of the Activated complex TwoEndothermic Kinds of Reactions Exothermic Absorb Energy Release Energy Heat + AB A +B A + B AB + Heat Heat is a reactant Heat is a product = Stability Break Bonds Bond formation + - H H (Most Effective Collisions) Entropy S Entropy is defined as the degree of randomness, disorder, chaos (s) – (l) – (aq) – (g) Gibbs Free energy G A reaction will always proceed spontaneously if the sign for Gibbs free energy is (-) The two conditions that favor a are : G G low energy - H Exothermic high entropy + S LeChatelier’s principle States that when a system that is at equilibrium is placed under a stress, the systems equilibrium will shift in order to relieve the stress Inc. (T) : will always favor endothermic reactions Inc (P) : will always favor the side of less mole formation A+B C+D Equilibrium N2 3H2 NH3 HEAT X X X X X X X X X X X X X X X X CH4 3H2 CO2 H2O HEAT X X X X X X X X X x Acids & Acids 1. 2. 3. 4. 5. Sour Taste Reacts with certain metals on table J to yield H2(g) Great Electrolytes (Why?) Excellent Conductors of Electricity (Why?) Cause Acid/Base Indicators to change colors Bases Bases Bitter 2. Slimy 1. Great Electrolytes (Why?) 4. Excellent conductors of Electricity (Why?) 5. Cause Acid/Base Indicators to change colors 3. Acid reacts with Base to yield Salt and Water Called the “Neutralization Reaction” The Neutralization Reaction Acid + Base -------------> Salt + Water HCl + NaOH --------> NaCl + H2O What kind of Reaction do you See? Double Replacement The Hydrolysis of a Salt The Reverse Reaction Adding water to a salt! Water + Salt --------> Acid + Base H2O + NaCl -------------> HCl + NaOH Called “The Parent Acid and Base” Definitions of Acid and Base Arrhenius Acid Any substance that yields (H+) as the only positive ion in solution HCl ------------> H+ + Cl- HBr ------------> H+ + Br- Base Any Substance that yields (OH-) ion as the only (-) ion in solution (Recall: Goup I,II Metal with OH and NH4OH) NaOH --------> Na+ + OH- H3O+ ----------> H+ + HOH Ca(OH)2 -----> Ca+2 + 2OHNH4OH ----> NH4+ + OH- H3PO4 ---------> H+ + H2PO4- Reminder: Do not confuse Base with Alcohols! (Hydrocarbon-OH) H2PO4 - -------> H+ HPO4-2 H+ --------> + HPO4-2 + PO4 -3 CH3OH CH3CH2OH Amphoterism Any Substance That can act as either acid or base H3O+ ----------> H+ + HOH OH- HOH ------------> H+ + H3PO4 ---------> H+ + H2PO4HPO4-2 H2PO4 - -------> H+ + HPO4-2 --------> H+ + PO4 -3 Definitions of Acid and Base Bronsted - Lowery Acid Proton (H+) Donor Base Proton (H+) Acceptor H+ H+ H2O + H2O -------------> OH- + H3O+ Strong Acid (SA) ----------> H+ + Weak Base (WB) Weak Acid (WA) ------------> H+ + Strong Base (SB) Strong Acids and Strong Bases Strong Acids HCl HBr HI H2SO4 HNO3 StrongBases Group I M Ca, Sr, Ba with OH pH Scale pH Scale Is a scale that is used to measure if a substance is an acid or base Measures the Percent [H+] (The power of Hydrogen!) pH Scale ****_____ For every decrease in pH value, this represents a 10x Increase in [H+] 5 <---------- 6 <------------ 7 <----------- 8 10 x 10 x 10 x <------------------------------------------------------------1000 x pH Calculations Ksp (The ionization of H2O) H2O <-----------> H+aq + OH- aq Keq = [H+] [OH-] Fact KH2O = 1 x 10 -14 1 x 10-14 = [H+] [OH-] [H+] = 1 x 10-7 [OH-] = 1x 10-7 Calculate pH pH = -log[H+] pOH = -log[OH-] pH = 7 pOH = 7 pH + pOH = 14 pH calculations What are the pH values of the following? .1 M HCl .01 M HCl .001M HCl The Hydrolysis of a Salt! Remember (it is the reverse reaction of a Neutralization reaction) __________________ KCl + HOH -----> KOH + HCl How can we determine the pH of the resulting solution? Titration Def. A technique that is used to determine the strength of an unknown (acid or base) compared with a known (acid or base). (coef A) MAVA = MBVB (coef B) We need an acid base indicator: Phenolphthalein Acid clear Base Pink Titration Technique Steps. Slowly add base to flask (watch for a color change to pale pink) ***Do not go past the end point! (coef A) MAVA = MBVB (coef B) Titration Titration (coef A) MAVA = MBVB (coef B) Titration and Calculations (coef A) MAVA = MBVB (coef B) End Point Naming Acids This is Review! Binary (2 elements) 1. Always starts with “Hydro” 2. Name the Non-Metal (Chlorine) 3. Drop the ending, add ic acid HCl Hydrochloric Acid HBr Hydrobromic Acid HI Hydroiodic Acid Tiernary (3 elements) M(PI) ate – ic H2SO4 Sulfuric Acid HNO3 Nitric Acid M(PI) ite - ous HNO2 Sulfurous Acid H2SO3 Nitrous Acid Assigning oxidation numbers 1. 2. 3. 4. 5. 6. Metals in group 1 have (+1) ox #, group 2 metals (+2) Any single “Pure” element = 0 Hydrogen is always (+1) except in metal hydride (-1) LiH Oxygen is always (-2) exceptions: With flourine (flouide) +2 OF2 In Peroxides (-1) H2O2 The sum of all oxidation #’s must = 0 The sum of all Polyatomic ions must equal the charge of that ion Assigning oxidation numbers Binary Compounds _____ HCl _______ MgCl 1. Start with the Non Metal 2. Finish with the Metal 3. Sum up must = 0 Assigning oxidation numbers Ternary Compounds _____ H2SO4 __________ Mg(NO3)2 1. Start with the Non Metal (Oxygen) 2. Go to the Metal (H) 3. finish up in the middle 4. Sum up must = 0 Redox Reactions (reactions where both Oxidation and Reduction take place) 1. Oxidation Reduction Loss of Electrons The gain of Electrons Half Reactions Mg0 Mg+2 + 2e(product) Half Reaction 2e- + Mg+2 Mg0 (reactant) Causes the Reduction of the other elements Causes the other species to be Oxidized. Acts as a REDUCING AGENT (R.A.) Acts as the Oxidation Agent (O.A.) Writing half reactions determine the Ox / red ra / oa Steps (Now this is Doc’s Method! …..Capisco?) 1. Assign the Ox #’s 2. Record the changes 3. Record e- loss / e- gain 4. Determine the species that is oxidized (RA) and reduced (OA) 5. Balance if unequal Ca + Cl2 CaCl2 ***HHH___ All Redox Reactions must demonstrate conservation of both Mass and Charge Electrochemical cell Spontaneous cell (battery) Voltaic cell, galvanic cell Al Cu (****Chemical energy electrical ****) Remember: A RED CAT IS AN OX and…….A RED CAT GETS FAT!!!!!! TABLE J Electrochemical cell Spontaneous cell (battery) Voltaic cell, galvanic cell Electrochemical cell Spontaneous cell (battery) Voltaic cell galvanic cell Electrochemical cell Spontaneous cell (battery) Voltaic cell galvanic cell Electrochemical cell Spontaneous cell (battery) Voltaic cell, galvanic cell Electrolytic cell non-spontaneous cell (need a power source) electroplating electrolysis (****electrical chemical energy****) Electrolytic cell non-spontaneous cell (need a power source) electroplating electrolysis (****electrical chemical energy****) Electrolytic cell non-spontaneous cell (need a power source) electroplating electrolysis of water!!! Electrolytic cell non-spontaneous cell (need a power source) electroplating electrolysis of water!!! Electrolytic cell non-spontaneous cell (need a power source) electrolysis of water!!! 2H2O 2H2 + O2 Hydrocarbon series Alkanes Alkenes All end in -ane All end in –ene General formula CnH2n+2 General Formula CnH2n Identify by the C-C bone (single) Saturated Hydrocarbons See C=C double bond Hydrocarbon series Alkynes All end with –yne General Formula of CnH2n-2 Identify the triple bond Hydrocarbon series Cyclic Hydrocarbons Also called Aromatic Hydrocarbons Also called Benzene series General formula is CnH2n-6 Benzene C6H6 Hydrocarbon series Cyclic Hydrocarbons 7 8 Toluene C H Also called Methyl Benzene Naming hydrocarbons Steps 1. Identify the longest C-C continuous chain Naming hydrocarbons Steps 2. Look for any C=C or C=C Bonds and identify by the lowest Carbon number location Naming hydrocarbons Naming hydrocarbons Naming hydrocarbons Step 3. Look for any Alkyl side chains: (Alkyl groups are Alkanes less 1 Hydrogen) Alkane Alkyl Dienes – contain 2 double bonds Functional Group - replaces a H atom of a hydrocarbon - an atom (or group of atoms) that give an organic compound specific chemical and physical properties (the following slides discuss functional groups for reference, I will show you how to correlate with your reference table O) R = an atom or group of atoms in a Hydrocarbon chain Organic Halides (Halocarbons) X = a Halogen (Group 17) RNaming Rule Indicate position X of halogen on longest hydrocarbon chain. Alcohols R-OH Naming Rule Indicate position of -OH group on carbon chain. Change “e” of alkane name to “ol.” Ethers R–O–R Naming Rule Name each R group and tack-on “ether.” Aldehydes and Ketones Naming Rules Aldehydes: Change “e” of alkane name to “al” Ketones: Indicate position of carbonyl Change “e” of alkane to “one” Organic Acids Naming Rules Change alkane “e” to “oic acid” Organic Acids Esters O R – C – O – R’ Naming Rules 1. Name R’ alcohol group 2. Change R alkane “e” to “oate” ***HHH______ Made from combining an Acid and an Alcohol Esters Amines R’ R – N – R’’ Naming Rules Label position of N. Change “e” of alkane name to “amine.” Amides O R’ R–C–N–H Naming Rule Change “e” of R alkane name to “amide” Types of organic reactions Adding a halogen to an unsaturated hydrocarbon (alkene / alkyne) Yields 1 product! + Br2 Types of organic reactions Adding a halogen to a saturated hydrocarbon (alkane) Yields 2 products, one an acid! + Br2 Br + HBr A reaction that always yields Ethanol and CO2! Recall: anaerobic Respiration?? A burn reaction (always needs O2) and always yields CO2 and H2O Making of Soap, always yields an Alcohol (Glycerol) and Soap! Look for the 3 NaOH bases!!!! Making Esters Acid + Alcohol yields an Ester and Water + C C ethanol + H2 O Making Esters Acid + Alcohol yields an Ester and Water Note the Esters 1. fragrances, sweet smells (bananas) Naming revisit 1. Start with the Alcohol Side 2. Finish with the Acid side The making of polymers 1. Natural – proteins, polysaccharides 2. Artificial – plastics, nylons, rayon Addition Polymerization Addition Polymerization Fact! 1. A Transmutation is any alteration in the nucleus 2. There are no stable isotopes above element #83 1. Elements that are unstable will emit energy in the form of Radiation 2. Called Radioisotopes 3. Stability is based on the P:N Ratio Types of Particles that are Emitted Particle Type Symbol Mass Charge Penetratin g Power Alpha 2He α 4 2 Low , β- 0 -1 Moderate Positron 0 , β+ e +1 0 +1 Moderate Gamma 0 0γ 0 0 High X-ray Beta -1e 0 4 The Effects of an Electric field on Charged Particles 2 Types of transmutations 1. Natural A type of Decay that occurs naturally therefore you will see only 1 reacting nuclei that will undergo a change 1. Alpha ( )Decay 2. Beta ( )Decay 3. Positron ( ) emission 4. Gamma ( ) decay Artificial A type of Decay that does not occur naturally, therefore you will see 2 nuclei reacting 2 Types 1. Fusion 2. Fission Natural Decay and Writing Nuclear Equations Alpha Notice several things about it: 1) The atom on the left side is the one that splits into two pieces. 2) One of the two atoms on the right is ALWAYS an alpha particle. 3) The other atom on the right ALWAYS goes down by two in the atomic number and four in the mass number. More Examples of Alpha decay More Examples of Alpha decay Check it and compare the three points to the example. Keep in mind that this equation shows the left-hand side splitting into the two pieces shown on the right-hand side. OK, write the alpha decay equations for these five nuclides. Beta decay and writing equations Beta decay practice Here's your first set of exercises. Write out the full beta decay equation. Then click the link to see the answers. Beta decay practice Here's your first set of exercises. Write out the full beta decay equation. Then click the link to see the answers. Positron emission 37 ------> K 19 0 + e +1 + B, 37 Ar 18 +1 0 e Pet scan (positron emission) Gamma decay No change in Mass or Charge Artificial Decay The conversion of matter into energy Fusion A reaction where 2 lite nuclei (alike) are joined together to make a heavier nuclei. Ex: Reactions on the Sun Good: Yields A lot of Energy Bad: Requires a lot of energy to run Fuse 2 like nuclei (repel) Need particle accelerators to inc. KE Synchroton Cyclotron Fission The splitting of heavy nuclei (bombard with high energy Neutrons 1 0N ) into a smaller nuclei Good: Yields a lot of energy Bad: Produces a lot of Radioactive waste (disposal issues and half life) Fusion + 1H1 -----> 1H2 + +1e0 + energy 1 + H2 -------> 3 H He 1 1 2 1H 1 Fission 1 + n 0 235 ------> Ba142 + U 92 56 91 + 3 n1 + ENERGY Kr 36 0 1 + n 0 235 ------> Xe143 + U 92 54 90 + 3 n1 + ENERGY Sr 38 0 Half-life The time that it takes for a substance (radioactive) to lose ½ its mass. A. Determine the Age of rocks (Carbon Dating) During Half-Life Problems Remember: 1. the longer the half-life, the SLOWER the decay (Dangerous) 2. The shorter the half-life, the faster the decay (used in medicine) Calculating the half-life You will need to know 5 things 1. Total time 2.½ life time 3.# of half life 4.Initial Mass 5.Final Mass If starting with Initial Mass If asked what fraction remains? Always start with (1) # of Half lifes # of Half lifes 128g 1 1 64g 1/2 2 32g 3 16g X 4 8g 5 4g 6 2g 7 1g ÷ X 1/4 1/8 1/16 1 2 3 4 ÷ Half-Life Initial Mass Total Time ½ life time = # of ½ lifes 2 # 2 Final Mass 1) The half-life of Zn-71 is 2.4 minutes. If one had 100.0 g at the beginning, how many grams would be left after 7.2 minutes has elapsed? 12.5g remaining 2) Os-182 has a half-life of 21.5 hours. How many grams of a 10.0 gram sample would have decayed after exactly three half-lives? 8.75g decayed, 1.25g remain 3) At time zero, there are 10.0 grams of W-187. If the half-life is 23.9 hours, how much will be present at the end of one day? Two days? Seven days? 1 day = 5.00g, 2 days = 2.50g, 7 days = .078g Uses of radioisotopes Carbon Dating – C14 no longer taken in by a dead organism Ratio of U238/Pb206 to date rocks I131 – used to study thyroid conditions Co60 – emits large amounts of gamma radiation. Used in treating cancers like Prostate Co60 and Cs137 – emit gamma rays, used to kill Anthrax Bacilli Tc43 –used to treat cancerous tumors, absorbed by cancer cells