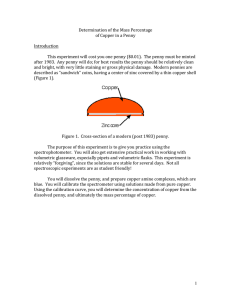
Atoms in a Pre-1982 Copper Penny
advertisement

Atoms in a Pre-1982 Copper Penny Name __________________________________ Period 1 2 3 4 5 6 Date ______________ 7 Background Information: All pennies that were made before 1982 were pure copper. After 1982, pennies were made with a disc of zinc on the inside and a copper coating on the outside. The diameter of one atom of copper is 2.7 x 10-10m. The mass of one copper atom is 1.05 x 10-22g. http://www.chemicool.com/elements/copper.html#radius Predict: How many atoms would you predict are contained in one pre-1982 penny? ________________ Activity: 1. Use the quad-beam balance to measure the exact mass in grams of 1 pre-1982 copper penny. ____________ (Record to the nearest 0.001g.) 2. Use a ruler to measure the exact diameter in centimeters across the middle of the penny. ____________ (Record to the nearest 0.01cm.) 3. Use the background information and your measured values to determine the number of copper atoms that could fit in a straight line across the middle of the penny. Show all your calculations using DA and circle your answer below. NOT to Scale. 4. Again, use the background information and your measured values to determine the TOTAL number of atoms in the penny. Show all your calculations using DA and circle your answer below. Hint: Use mass to figure it out. 5. How close was your prediction to the actual number of atoms in the penny? 6. Use the milliliter graduated cylinder to measure the volume of a penny. a. Add exactly 20mL to the graduated cylinder. b. Insert 10 pennies and record the new volume in mL. _______________ c. Calculate how much the volume went up by adding the pennies. Show your math. d. Divide the volume from part c. by 10 to get the volume of ONE penny. Show your math. 7. Calculated the density of the penny. Show your math. 8. The accepted density of copper is 8.96 g/mL. Calculate your percent error. Show your math. 9. Write a 4-5 sentence paragraph summarizing your results and what you have learned about the size of an atom. ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________








![2014 LABORATORY MANUAL [ ]](http://s2.studylib.net/store/data/014300427_1-2884b78f05395e8f0039d5ab84c4c748-300x300.png)