6. Neoplasia new 2 molecu
advertisement

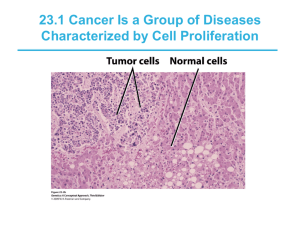
MOLECULAR BASIS of CANCER • NON-lethal genetic damage • A tumor is formed by the clonal expansion of a single precursor cell (monoclonal) • Four classes of normal regulatory genes – PROTO-oncogenes – Oncogenes Oncoproteins – DNA repair genes – Apoptosis genes • Carcinogenesis is a multistep process TRANSFORMATION & PROGRESSION • • • • • • • • Self-sufficiency in growth signals Insensitivity to growth-inhibiting signals Evasion of apoptosis Defects in DNA repair: “Spell checker” Limitless replicative potential: Telomerase Angiogenesis Invasive ability Metastatic ability Oncogenes Cell cycle Apoptosis Angiogenesis Tumor Suppressor Inv. and Mets 3 Hanahan and Weinberg, Cell 100: 57, 2000 ONCOGENES • Oncogenes are mutated forms of cellular proto-oncogenes. • Proto-oncogenes code for cellular proteins which regulate normal cell growth and differentiation. 4 Five types of proteins encoded by proto-oncogenes participate in control of cell growth: Class I: Growth Factors Class II: Receptors for Growth Factors and Hormones Class III: Intracellular Signal Transducers Class IV: Nuclear Transcription Factors Class V: Cell-Cycle Control Proteins 5 Functions of Cellular Proto-Oncogenes 1. Secreted Growth Factors 2. Growth Factor Receptors 3. Cytoplasmic Signal Transduction Proteins 4. Nuclear Proteins: Transcription Factors 5. Cell Growth Genes 6 ONCOGENES • Are MUTATIONS of NORMAL genes (PROTO-oncogenes) – Growth Factors – Growth Factor Receptors – Signal Transduction Proteins (RAS) – Nuclear Regulatory Proteins – Cell Cycle Regulators • Oncogenes code for Oncoproteins Mutations that confer these properties fall into two categories • Oncogene • : a cancer-causing gene that has been mutated to cause an increase in • activity, or the activity becomes constitutive, or a new activity is acquired. • -a mutation in a single allele is sufficient to transform cells (dominant). • -originally identified as viral proteins that resembled normal human proteins. • -the term "proto-oncogene" refers to the normal protein that has not been mutated • tumor Suppressor gene • : cancer-causing gene that has been mutated to cause a loss of activity. • -mutations are required in both alleles to transform cells (recessive) 4 types of genetic mutations that contribute to cancer 1 2 4 3 • Categories of oncogenes • A. Growth factors • -generally not directly involved transformation, but increased expression seen as part of • an autocrine loop due to changes in other steps in the same pathway growth factor receptors • -They are transmembrane proteins with an external ligand binding domain and an • internal tyrsosine kinase domain. • -oncogenic mutations can result in dimerization and activation in the absence of • ligand • -more commonly, increased activity is a result of overexpression of receptors Growth factor receptors • They are transmembrane proteins with an external ligand binding domain and an • internal tyrsosine kinase domain. • -Oncogenic mutations can result in dimerization and activation in the absence of • ligand • -More commonly, increased activity is a result of overexpression of receptors. signal transducers • -Activated directly or indirectly by growth factor receptors • -Activation of signal transducers triggers a phosporylation cascade that ultimately • results in changes in gene expression at the transcriptional level. • -mutations in RAS • , a GTPase, are the most common oncogenic abnormality in tumors • -failure to hydrolyze GTP locks RAS in its active form. Transcription factors • -Transcription factors contain DNA binding domains. • Sequences • Regulate expression of genes essential for passage through the cell cycle, or • regulation of apoptosis. • - Normal CELL CYCLE Phases INHIBITORS: Cip/Kip, INK4/ARF Tumor (really growth) suppressor genes: p53 cyclins and cyclin-dependent kinases • -cyclins are only expressed at specific stages of the cell cycle • -cyclin-dependent kinases are expressed constitutively, but must bind cyclins for • activation; phosphorylation of target proteins essential for progression through • cell cycle Regulation of G1/S cell cycle transition Cell cycle arrest at G1/S (in response to DNA damage or other stressors) is medicated through which gene? p53 (levels of p53 under negative regulation by MDM2 and p14 ARF) • a second level of control is achieved by CDK inhibitors • -p21 family (broad specificity) and the INK4 (p16) family (CDK4/6 • specific) • -overexpression of cyclin D and CDK4 common. • -phosphorylate and inactivate • Rb Category PROTOOncogene Mode of Activation Associated Human Tumor GFs PDGF-β chain SIS Fibroblast HST-1 growth factors INT-2 TGFα HGF Overexpression Astrocytoma Osteosarcoma Overexpression Stomach cancer Amplification Bladder cancer TGFα Breast cancer Melanoma Overexpression Astrocytomas HGF Hepatocellular carcinomas Overexpression Thyroid cancer Category PROTOOncogene Mode of Activation Associated Human Tumor GF Receptors EGF-receptor family ERB-B1 (ECFR) ERB-B2 Overexpression Amplification Squamous cell carcinomas of lung, gliomas Breast and ovarian cancers CSF-1 receptor FMS Point mutation Leukemia Receptor for neurotrophic factors PDGF receptor RET Point mutation PDGF-R Overexpression Multiple endocrine neoplasia 2A and B, familial medullary thyroid carcinomas Gliomas Receptor for stem cell (steel) factor KIT Point mutation Gastrointestinal stromal tumors and other soft tissue tumors Category PROTOOncogene Mode of Activation Associated Human Tumor Signal Transduction Proteins GTP-binding Nonreceptor tyrosine kinase K-RAS Point mutation Colon, lung, and pancreatic tumors H-RAS Point mutation Bladder and kidney tumors N-RAS Point mutation Melanomas, hematologic malignancies ABL Translocation Chronic myeloid leukemia Acute lymphoblastic leukemia RAS signal transduction BRAF Point mutation Melanomas WNT signal transduction β-catenin Point mutation Hepatoblastomas, hepatocellular carcinoma PROTOOncogene Category Nuclear Regulatory Proteins Transcrip. C-MYC activators N-MYC L-MYC Mode of Activation Associated Human Tumor Translocation Burkitt lymphoma Amplification Neuroblastoma, small cell carcinoma of lung Amplification Small cell carcinoma of lung 2) Activation Growth-Promoting Oncogenes Which signal transduction pathway is continuously activated by mutant RAS? MAP kinase pathway Point mutations of ras are seen in what % of all human malignancies? 15-20% Tumor supressor gene • . Tumor suppressor were originally identified as inherited mutations that confer a • predisposition to cancer (familial form). • -inheritance is dominant, meaning a single defective allele is sufficient to confer • the predisposition • • • • • • • • • • Inactivation of tumor suppressors can occur Sporadically -sequential inactivation of both alleles in somatic cells You may hear the term haploinsufficiency , which refers to inactivation of a single allele contributing to malignancy. -usually not the initiating event, but exacerbating. Viral inactivation -HPV expresses proteins that inhibit Rb and p53 function. P53 and RAS p53 • Activates DNA repair proteins • Sentinel of G1/S transition • Initiates apoptosis • Mutated in more than 50% of all human cancers RAS • H, N, K, etc., varieties • Single most common abnormality of dominant oncogenes in human tumors • Present in about 1/3 of all human cancers RB gene • a.Loss of RB function confers a predisposition to retinoblastoma. • occurs in both the familial form (early onset) and sporadic fromthe basis for tissue specificity of some tumor suppressors is unknown, but • presumably is due to the transcriptional profile of the tissue, determined by tissue • function P53 • p53 is the most commonly mutated gene in tumors • -over 50% of all tumors lack functional p53 • • Li-Fraumeni syndrome • : inheritance of a single defective copy of p53 results in a • predisposition to a wide spectrum of cancers. • -p53 is a transcription factor. 1: Failure of DNA Repair (acquired) Normal function of p53 is to upregulate activity of which 2 genes to allow repair of DNA? p21 GADD45 • Unlike Rb, p53 inhibits G1 progression only in response to DNA damage • -normally p53 is very unstable, due to proteolytic degradation triggered by • mdm2 • . • -p53 is phosphorylated in response to DNA damage; mdm2 no longer binds p53 • -p53 upregulates expression of p21, which in turn inhibits G1/S CDKs. • c. In response to excessive DNA damage, p53 can trigger apoptosis • Some other tumor suppressors found to be inactivated in tumors inhibit proliferation by • various mechanisms: • -APC: degradation of • b • -catenin, a transcriptional activator anchored to E-cadherins • -NF-1: activates GTPase activity of ras • -TGF• b • receptor: a tyrosine kinase that upregulates expression of CDK inhibitors • - • -PTEN: dephosphorylates inositol phospholipids, which act as docking sites for • intracellular signaling proteins • VHL: transcriptional elongation • -WT-1: transcriptional regulator MYC • Encodes for transcription factors • Also involved with apoptosis Tumor (really “GROWTH”) suppressor genes • • • • • • • • • • • TGF-β COLON E-cadherin STOMACH NF-1,2 NEURAL TUMORS APC/β-cadherin GI, MELANOMA SMADs GI RB RETINOBLASTOMA P53 EVERYTHING!! WT-1 WILMS TUMOR p16 (INK4a) GI, BREAST BRCA-1,2 BREAST KLF6 PROSTATE Evasion of APOPTOSIS •BCL-2 •p53 •MYC DNA REPAIR GENE DEFECTS • DNA repair is like a spell checker • HNPCC (Hereditary Non-Polyposis Colon • • • • Cancer [Lynch]): TGF-β, β-catenin, BAX Xeroderma Pigmentosum: UV fixing gene Ataxia Telangiectasia: ATM gene Bloom Syndrome: defective helicase Fanconi anemia LIMITLESS REPLICATIVE POTENTIAL • TELOMERES determine the limited number of duplications a cell will have, like a cat with nine lives. • TELOMERASE, present in >90% of human cancers, changes telomeres so they will have UNLIMITED replicative potential TUMOR ANGIOGENESIS • Q: How close to a blood vessel must a cell be? • A: 1-2 mm • Activation of VEGF and FGF-b • Tumor size is regulated (allowed) by angiogenesis/anti-angiogenesis balance TRANSFORMATION GROWTH BM INVASION ANGIOGENESIS INTRAVASATION EMBOLIZATION ADHESION EXTRAVASATION METASTATIC GROWTH etc. Invasion Factors • Detachment ("loosening up") of the tumor cells from each other • Attachment to matrix components • Degradation of ECM, e.g., collagenase, etc. • Migration of tumor cells METASTATIC GENES? • NM23 • KAI-1 • KiSS CHROMOSOME CHANGES in CANCER • TRANSLOCATIONS and INVERSIONS • Occur in MOST Lymphomas/Leukemias • Occur in MANY (and growing numbers) of NONhematologic malignancies also Malignancy Chronic myeloid leukemia Translocation (9;22)(q34;q11) Affected Genes Ab1 9q34 bcr 22q11 Acute leukemias (AML and ALL) (4;11)(q21;q23) AF4 4q21 MLL 11q23 (6;11)(q27;q23) AF6 6q27 MLL 11q23 Burkitt lymphoma (8;14)(q24;q32) c-myc 8q24 IgH 14q32 Mantle cell lymphoma (11;14)(q13;q32) Cyclin D 11q13 IgH 14q32 Follicular lymphoma (14;18)(q32;q21) IgH 14q32 bcl-2 18q21 T-cell acute lymphoblastic leukemia (8;14)(q24;q11) c-myc 8q24 TCR-α 14q11 (10;14)(q24;q11) Hox 11 10q24 TCR-α 14q11 Ewing sarcoma (11;22)(q24;q12) Fl-1 11q24 Carcinogenesis is “MULTISTEP” • NO single oncogene causes cancer • BOTH several oncogenes AND several tumor suppressor genes must be involved • Gatekeeper/Caretaker concept –Gatekeepers: ONCOGENES and TUMOR SUPPRESSOR GENES –Caretakers: DNA REPAIR GENES • Tumor “PROGRESSION” – ANGIOGENESIS – HETEROGENEITY from original single cell Carcinogenesis: The USUAL (3) Suspects • Initiation/Promotion concept: – BOTH initiators AND promotors are needed – NEITHER can cause cancer by itself –INITIATORS (carcinogens) cause MUTATIONS – PROMOTORS are NOT carcinogenic by themselves, and MUST take effect AFTER initiation, NOT before –PROMOTORS enhance the proliferation of initiated cells Q: WHO are the usual suspects? • Inflammation? • Teratogenesis? • Immune Suppression? • Neoplasia? • Mutations? A: The SAME 3 that are ALWAYS blamed! •1) Chemicals •2) Radiation •3) Infectious Pathogens CHEMICAL CARCINOGENS: INITIATORS • DIRECT • “PRO”CARCINOGENS • β-Propiolactone Dimeth. sulfate • Diepoxybutane Anticancer drugs • (cyclophosphamide, chlorambucil, nitrosoureas, and others) • Acylating Agents • • • • – 1-Acetyl-imidazole – Dimethylcarbamyl chloride Polycyclic and Heterocyclic Aromatic Hydrocarbons Aromatic Amines, Amides, Azo Dyes Natural Plant and Microbial Products – – – – – Aflatoxin B1 Hepatomas Griseofulvin Antifungal Cycasin from cycads Safrole from sassafras Betel nuts Oral SCC CHEMICAL CARCINOGENS: INITIATORS • OTHERS • • • • • • • Nitrosamine and amides (tar, nitrites) Vinyl chloride angiosarcoma in Kentucky Nickel Chromium Insecticides Fungicides PolyChlorinated Biphenyls (PCBs) CHEMICAL CARCINOGENS: PROMOTORS • • • • HORMONES PHORBOL ESTERS (TPA), activate kinase C PHENOLS DRUGS, many “Initiated” cells respond and proliferate FASTER to promotors than normal cells RADIATION CARCINOGENS • UV: BCC, SCC, MM (i.e., all 3) • IONIZING: photons and particulate – Hematopoetic and Thyroid (90%/15yrs) tumors in fallout victims – Solid tumors either less susceptible or require a longer latency period than LEUK/LYMPH – BCCs in Therapeutic Radiation VIRAL CARCINOGENESIS • • • • • HPV SCC EBV Burkitt Lymphoma HBV HepatoCellular Carcinoma (Hepatoma) HTLV1 T-Cell Malignancies KSHV Kaposi Sarcoma H. pylori CARCINOGENESIS • 100% of gastric lymphomas (i.e., M.A.L.T.-omas) • Gastric CARCINOMAS also! HOST DEFENSES • IMMUNE SURVEILLENCE CONCEPT • • • • CD8+ T-Cells NK cells MACROPHAGES ANTIBODIES CYTOTOXIC CD8+ T-CELLS are the main eliminators of tumor cells How do tumor cells escape immune surveillance? • Mutation, like microbes • ↓ MHC molecules on tumor cell surface • Lack of CO-stimulation molecules, e.g., (CD28, ICOS), not just Ag-Ab recognition • Immunosuppressive agents • Antigen masking • Apoptosis of cytotoxic T-Cells (CD8), i.e., the damn tumor cell KILLS the T-cell! Effects of TUMOR on the HOST • • • • • Location anatomic ENCROACHMENT HORMONE production Bleeding, Infection ACUTE symptoms, e.g., rupture, infarction METASTASES CACHEXIA • • • • • Reduced diet: Fat loss>Muscle loss Cachexia: Fat loss AND Muscle loss TNF (α by default) IL-(6) PIF (Proteolysis Inducing Factor) PARA-Neoplastic Syndromes •Endocrine (next) • Nerve/Muscle, e.g., myasthenia w. lung ca. • Skin: e.g., acanthosis nigricans, dermatomyositis • Bone/Joint/Soft tissue: HPOA (Hypertrophic Pulmonary OsteoArthropathy) • Vascular: Trousseau, Endocarditis • Hematologic: Anemias • Renal: e.g., Nephrotic Syndrome ENDOCRINE Cushing syndrome Small cell carcinoma of lung ACTH or ACTH-like substance Pancreatic carcinoma Neural tumors Syndrome of inappropriate antidiuretic hormone secretion Small cell carcinoma of lung; intracranial neoplasms Antidiuretic hormone or atrial natriuretic hormones Hypercalcemia Squamous cell carcinoma of lung Parathyroid hormone-related protein (PTHRP), TGF-α, TNF, IL-1 Breast carcinoma Renal carcinoma Adult T-cell leukemia/lymphoma Ovarian carcinoma Hypoglycemia Fibrosarcoma Insulin or insulin-like substance Other mesenchymal sarcomas Hepatocellular carcinoma Carcinoid syndrome Bronchial adenoma (carcinoid) Serotonin, bradykinin Pancreatic carcinoma Gastric carcinoma Polycythemia Renal carcinoma Cerebellar hemangioma Hepatocellular carcinoma Erythropoietin GRADING/STAGING • GRADING: HOW “DIFFERENTIATED” ARE THE CELLS? • STAGING: HOW MUCH ANATOMIC EXTENSION? TNM • Which one of the above do you think is more important? WELL? (pearls) MODERATE? (intercellular bridges) POOR? (WTF!?!) GRADING for Squamous Cell Carcinoma ADENOCARCINOMA GRADING Let’s have some FUN! LAB DIAGNOSIS • BIOPSY • CYTOLOGY: (exfoliative) • CYTOLOGY: (FNA, Fine Needle Aspirate) IMMUNOHISTOCHEMISTRY • Categorization of undifferentiated tumors • Leukemias/Lymphomas • Site of origin • Receptors, e.g., ERA, PRA