Solar1
advertisement

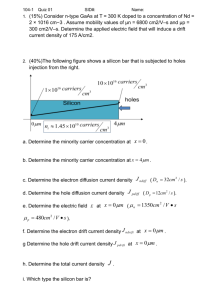
Solar Photovoltaic Physics Basic Physics and Materials Science of Solar Cells Original Presentation by J. M. Pearce, 2006 Email: profpearce@gmail.com What are Photovoltaics? • Photovoltaic (PV) systems convert light energy directly into electricity. • Commonly known as “solar cells.” • The simplest systems power the small calculators we use every day. More complicated systems will provide a large portion of the electricity in the near future. • PV represents one of the most promising means of maintaining our energy intensive standard of living while not contributing to global warming and pollution. A Brief History Photovoltaic Technology • • • • 1839 – Photovoltaic effect discovered by Becquerel. 1870s – Hertz developed solid selenium PV (2%). 1905 – Photoelectric effect explained by A. Einstein. 1930s – Light meters for photography commonly employed cells of copper oxide or selenium. • 1954 – Bell Laboratories developed the first crystalline silicon cell (4%). • 1958 – PV cells on the space satellite U.S. Vanguard (better than expected). Things Start To Get Interesting... • mid 1970s – World energy crisis = millions spent in research and development of cheaper more efficient solar cells. • 1976 – First amorphous silicon cell developed by Wronski and Carlson. • 1980’s - Steady progress towards higher efficiency and many new types introduced • 1990’s - Large scale production of solar cells more than 10% efficient with the following materials: – Ga-As and other III-V’s – CuInSe2 and CdTe – TiO2 Dye-sensitized – Crystalline, Polycrystalline, and Amorphous Silicon • Today prices continue to drop and new “3rd generation” solar cells are researched. Types of Solar Photovoltaic Materials Photovoltaic Materials Electronic Structure of Semiconductors • Silicon • Group 4 elemental semiconductor • Silicon crystal forms the diamond lattice • Resulting in the use of four valence electrons of each silicon atom. Crystalline Silicon Amorphous Silicon Solar PV Materials: Crystalline & Polycrystalline Silicon • Advantages: – High Efficiency (14-22%) – Established technology (The leader) – Stable • Disadvantages: – Expensive production – Low absorption coefficient – Large amount of highly purified feedstock Amorphous Silicon Advantages: • High absorption (don’t need a lot of material) • Established technology • Ease of integration into buildings • Excellent ecological balance sheet • Cheaper than the glass, metal, or plastic you deposit it on Disadvantages: • Only moderate stabilized efficiency 710% • Instability- It degrades when light hits it – Now degraded steady state How do they work? The physics view Band Theory Ef Eg Ef Metal Insulator Ef Semiconductor • There are 3 types of materials in Band Theory, which are differentiated by their electronic structure: – insulators, – conductors, and – semiconductors. Energy Bands in a Semiconductor • Conduction Band – Ec – empty • Valence Band – Ev – full of electrons 3 Types of Semiconductors 1. Intrinsic 2. n-type 3. p-type • Types 2 and 3 are semiconductors that conduct electricity - How? – – by alloying semiconductor with an impurity, also known as doping carriers placed in conduction band or carriers removed from valence band. Note: Color Protocol Type 1: Intrinsic • Pure semiconductor (intrinsic): contains the right number of electrons to fill valence band, therefore, conduction band is empty. • Because electrons in full valence cannot move, the pure semiconductor acts like an insulator. Type 2: n-Type • n-type: current is carried by negatively charged electrons - How? – group 5 impurity atoms added to silicon melt from which is crystal is grown – 4/5 of outer electrons used to fill valence band – 1/5 left is then put into conduction band. These impurity atoms are called donors. Within conduction band the electrons are moving, therefore, crystal becomes a conductor Type 3: p-Type • p-Type: current carried by missing electron holes which act as positively charged particles. How? – group 3 added to silicon melt – need 4 out of 5 outer electrons but doping creates lack of electrons in valence band. – missing electrons, a.k.a holes, are used to carry current. What Carries the Current? • Prevailing charges are called the majority carriers – prevailing charge carrier in n-type: electrons – prevailing charge carrier in p-type: holes Creating a Junction • There are four main types of semiconductor junctions – – – – p-n p-i-n Schottcky barrier Heterojunction • Each has a built in potential p-n and p-i-n Junctions Vbi Ef Vbi Ef Schottky Barriers and Heterojunctions Semiconductor Junctions • All the junctions contain strong electric field • How does the electric field occur? – When two semiconductors come into contact, electrons near interface from n-type, transfer over to p-type, leaving a positively charged area – Holes from p-type by interface transfer over to n-type leaving a negatively charged area. – Because electrons and holes are swapped, a middle potential barrier with no mobile charges, is formed. – This potential barrier created does not let any more electrons or holes flow through. • Electric field pulls electrons and holes in opposite directions. Barrier Changes • Equilibrium means there is no net current • Reduced barrier height is called forward bias (positive voltage applied to p-side) – Result- increases current through diode • Increased barrier height is called reverse bias. – Result- decreases current to a very small amount.. Electric Currents in p-n Junction Under External Bias Diode I-V Characteristics Current in a Solar Cell • Output current = I = Il-Io [ exp(qV/kT)-1] – – – – Il=light generated current q = electric charge V = voltage k = Boltzman’s constant = 1.3807 × 10-23 J/K • When in open circuit (I=0) all light generated current passes through diode • When in short circuit (V=0) all current passes through external load 2 Important points: 1) During open circuit the voltage of open circuit, Voc = (kT/q) ln( Il/Io +1) 2) No power is generated under short and open circuit - but Pmax = VmIm=FFVocIsc I-V Curve for Solar Cells Fourth quadrant (i.e., power quadrant) of the illuminated I-V characteristic defining fill factor (FF) and identifying Jsc and Voc Light Absorption by a Semiconductor • • • Photovoltaic energy relies on light. Light → stream of photons → carries energy Example: On a clear day 4.4x1017 photons hit 1 m2 of Earth’s surface every second. • Eph()=hc/ =hf – h = plank’s constant = 6.625 x 10-34 J-s – = wavelength – c = speed of light =3 x 108 m/s – f = frequency • However, only photons with energy in excess of bandgap can be converted into electricity by solar cells. The Solar Spectrum The entire spectrum is not available to single junction solar cell Generation of Electron Hole Pairs with Light • Photon enters, is absorbed, and lets electron from VB get sent up to CB • Therefore a hole is left behind in VB, creating absorption process: electron-hole pairs. • Because of this, only part of solar spectrum can be converted. • The photon flux converted by a solar cell is about 2/3 of total flux. Generation Current • Generation Current = light induced electrons across bandgap as electron current • Electron current:= Ip=qNA – N = # of photons in highlighted area of spectrum – A = surface area of semiconductor that’s exposed to light • Because there is current from light, voltage can also occur. • Electric power can occur by separating the electrons and holes to the terminals of device. • Electrostatic energy of charges occurs after separation only if its energy is less than the energy of the electron-hole pair in semiconductor • Therefore Vmax=Eg/q • Vmax= bandgap of semiconductor is in EV’s, therefore this equation shows that wide bandgap semiconductors produce higher voltage. Direct vs Indirect Bandgap • Everything just talked about, where all energy in excess of bandgap of photons are absorbed, are called direct-bandgap semiconductors. • More complicated absorption process is the indirect-gap series – quantum of lattice vibrations, of crystalline silicon, are used in the conversion of a photon into electron-hole pair to conserve momentum there hindering the process and decreasing the absorption of light by semiconductor. The Solar Cell • Electric current generated in semiconductor is extracted by contacts to the front and rear of cell. Widely spaced thin strips (fingers) are created so that light is allowed through. • – these fingers supply current to the larger bus bar. • Antireflection coating (ARC) is used to cover the cell to minimize light reflection from top surface. • ARC is made with thin layer of dielectric material. Different Types of Photovoltaic Solar Cells Diffusion Drift Excitonic Diffusion • n-type and p-type are aligned by the Fermilevel • When a photon comes in n-type, it takes the place of a hole, the hole acts like an air bubble and “floats” up to the p-type • When the photon comes to the p-type, it takes place of an electron, the electron acts like a steel ball and “rolls” down to the n-type Diagram of p-n Junction and Resultant Band Structure Drift • There is an intrinsic gap where the photon is absorbed in and causes the electron hole pair to form. • The electron rises up to the top and drifts downwards (to n-type) • The hole drifts upwards (to p-type) Excitonic Solar Cell • Dye molecule – electron hole pair splits because it hits the dye – the electron shifts over to the electric conductor and the hole shifts to the hole conductor Power Losses in Solar Cells Recombination • Opposite of carrier generation, where electron-hole pair is annihilated • Most common at: – impurities – defects of crystal structure – surface of semiconductor • Reducing both voltage and current Series Resistance • Losses of resistance caused by transmission of electric current produced by the solar cell. • I-V characteristic of device: • I = Il-I0 [exp(qV+IRs / mkT) – 1] • m= nonideality factor Other Losses • Current losses- called collection efficiency, ratio b/w number of carriers generated by light by number that reaches the junction. • Temperature dependence of voltage – V decreases as T increases • Other losses – light reflection from top surface – shading of cell by top contacts – incomplete absorption of light Minimize Recombination Losses by Adapting the Device Tandem Cells Silver Grid Indium Tin Oxide p-a-Si:H Blue Cell i-a-Si:H n-a-Si:H p Green Cell i-a-SiGe:H (~15%) n p Red Cell i-a-SiGe:H (~50%) n Textured Zinc Oxide Silver Stainless Steel Substrate Schematic diagram of state-of-theart a-Si:H based substrate n-i-p triple junction cell structure. • Tandem cellseveral cells, – Top cell has large bandgap – Middle cell mid eV bandgap – Bottom cell small bandgap. Solar Photovoltaics is the Future Acknowledgements • This is the first in a series of presentations created for the solar energy community to assist in the dissemination of information about solar photovoltaics. • This work was supported from a grant from the Pennsylvania State System of Higher Education. • The author would like to acknowledge assistance in creation of this presentation from Heather Zielonka, Scott Horengic and Jennifer Rockage.