Metabolism: the Degradation and Synthesis of Living Cells
advertisement

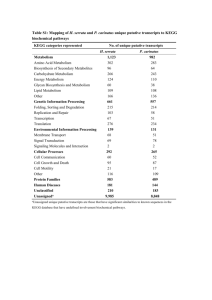
Instructors for Biochemistry II • Dr. Yongmei Qin (秦咏梅,Associate Professor, director of Biochemistry II) – Tel.:6275-8885 (office); Room 214, New Life Science Building; – E-mail: qinym@pku.edu.cn • Dr. Zengyi Chang (昌增益, Professor) – Tel: 6275-8822 (office); Room 204, New Life Science Building; – E-mail: changzy@pku.edu.cn Teaching Assistants for Biochemistry II • Ruiyu Kang (康瑞玉):6275-8056 Raymank@pku.edu.cn • Fangyuan Chen (陈方圆): faye@pku.edu.cn • Anastasia Ngozi(阿娜莎):62758056 anasha@pku.edu.cn Did you ever ask these questions? • How the nutrients that we are ingesting daily become part of our body and allow growth to occur (what is the fate of the sugar, fat, protein and nucleic acids that enter our body along with the food ?) • Why do we become fat by only eating sugar? • What is the molecular nature of the large number of genetic diseases? How can we find ways to prevent and treat them? • What does O2 do for us? And how is O2 produced by plants? • How do the various living organisms “produce” and “consume” energy? … Biochemistry II Metabolism: The totality of the transformation of biomolecules (matter) and energy Definition of Metabolism • The entire highly integrated and regulated network of chemical transformations (as stepwise metabolic pathways catalyzed by many enzymes) occurring in a living organism (through which cells extract energy and reducing power from its environment, as well as synthesize the building blocks of its macromolecules and then the macromolecules themselves). Coenzymes (vitamines) Amino acids carbohydrate hormones nucleotides Amino acids lipids 22nd edition designed by Dr. Donald E. Nicholson For maps of metabolic pathways see: http://www.iubmbnicholson.org/ metabolism is categorized into two types • Catabolism (biodegradation): larger molecules (nutrients and cell constituents) are broken down (often via exergonic reactions) to salvage (reuse) their components or/and to generate energy. • Anabolism (biosynthesis): The generation of biomolecules from simpler components via The Ying & Yang (often of Metabolism (Fuels) The Ying & Yang of Metabolism Exergonic Oxidation Biodegradation Output of energy Simpler Metabolites Complex Metabolites Input of energy Endergonic Reduction Biosynthesis Major Roles of Metabolism • Extract energy and reducing power from the environment (photosynthesis and oxidative degradation of nutrients). • Generation (interconversion) of all the biomolecules for a living organism. Thus comes the term “Dynamic Biochemistry” (Fuels) The role of Metabolism Extract energy and reducing power ATP: Energy currency Also for mobility, transport of nutrients and so on. Generate all biomolecules Classification of organisms based on trophic (“feed”) strategies • Autotrophs—synthesize all cellular components from simple inorganic molecules (e.g, H2O, CO2, NH3, H2S). • Heterotrophs—Derive energy from oxidation of organic compounds (made by autotrophs). Metabolism in various living organisms allow carbon, oxygen and nitrogen to be cycled in the biosphere. The cycling of matter is driven by the flow of energy in one direction through the biosphere! Metabolism allows the cycling of C/O and the flow of energy in the biosphere glucose Producers Consumers H2O Metabolism also allows the cycling of N in the biosphere (NH4+) NO3NO2- General Features of Metabolism • Occurs in specific cellular (tissue and organ) locations as a series of enzyme-catalyzed linear, branched or circular reactions, or pathways. • Highly coupled and interconnected (“Every road leads to Rome”). • Highly regulated (often reciprocally) to achieve the best economy (“Balanced supply and demand”). • The number of reactions is large (over 1000), however, the number of types of reactions is relatively small (what happens in animal respiration happens in plant photosynthesis). • Well conserved during evolution: reflecting the unity of the life phenomena (“what happens in bacteria happens in human being”). (乙酰辅酶A) General approaches for studying metabolism • Purification and Chemical characterization of metabolites; • Tracing the fates of certain biomolecules in living subjects (via such chemical labels as isotopes). • Isolation of genetic mutants having genetic defects. • Identification and characterization of enzymes. Issues for current and future investigation on metabolism • Continue to unveil new pathways and new regulation strategies of metabolism. • Studies on enzymes. • Observation of metabolic processes in intact living organisms (e.g., in the brains under various states) • Metabolism differences among various organisms or various states of the same organism (for diagnosing and treating such diseases as cancer, infections of bacteria or viruses, obesity, etc; to understand aging). • Appropriate and inappropriate nutrition. • Biotechnological application of knowledge learned from metabolic studies in medicine, agriculture and industry. • Nobel Prizes in revealing the Metabolism of living matter (1) • 1907, Eduard Buchner: cell-free fermentation. • 1922, Archibald B. Hill: production of heat in the muscle?; Otto Meyerhof: fixed relationship between the consumption of oxygen and the metabolism of lactic acid in the muscle. • 1923, Frederick Grant Banting, John James Richard Macleod: discovery of insulin. • 1929, Arthur Harden, Hand von Euler-Chelpin: fermentation of sugar and fermentative enzymes. • 1929, Christiaan Eijkman: antineuritic vitamin; Sir Frederick Gowland Hopkins: growth-stimulating vitamins. • 1931, Otto Heinrich Warburg: nature and mode of action of the respiratory enzyme. Nobel Prizes in revealing the Metabolism of living matter (2) • 1934, George Hoyt Whipple, George Richards Minot, William Parry Murphy: liver therapy in cases of anaemia. • 1937, Albert Szent-Gyorgyi: biological combustion, vitamin C and the catalysis of fumaric acid. • 1943, Henrik Carl Peter Dam: discovery of vitamin K; Edward Adelbert Doisy: chemical nature of vitamin K. • 1947, Carl Cori and Gerty Cori: catalytic conversion of glycogen; Bernardo Houssay: hormone of the anterior pituitary lobe in the metabolism of sugar. • 1950, Edward Calvin Kendall, Tadeus Reichstein,Philip Showalter Hench: hormones of the adrenal cortex, their structure and biological effects. • 1953, Hans Krebs: citric acid cycle; Fritz Lipmann: role of co-enzyme A in metabolism. • 1955, Axel Hugo Theodor Theorell: nature and mode of action of oxidation enzymes“. Nobel Prizes in revealing the Metabolism of living matter (3) • 1961, Melvin Calvin: carbon dioxide assimilation in plants. • 1964, Konrad Bloch, Feodor Lynen: cholesterol and fatty acid metabolism. • 1971, Earl W. Sutherland, Jr.: mechanisms of the action of hormones. • 1978, Peter Mitchell: chemiosmotic theory of biological energy transfer. • 1982, Sune K. Bergström, Bengt I. Samuelsson, John R. Vane: prostaglandins and related biologically active substances. • 1985. Michael S. Brown, Joseph L. Goldstein: regulation of cholesterol metabolism. Nobel Prizes in revealing the Metabolism of living matter (4) • 1988, Sir James W. Black, Gertrude B. Elion, George H. Hitchings: principles for drug treatment. • 1988, Johann Deisenhofer, Robert Huber, Hartmut Michel: photosynthetic reaction centre. • 1992, Edmond H. FischerEdwin G. Krebs: reversible protein phosphorylation as a biological regulatory mechanism. • 1994, Alfred G. GilmanMartin Rodbell: G-proteins and the role of these proteins in signal transduction in cells. • 1997, Paul D. Boyer, John E .Walker: synthesis of ATP. • 1998, Robert F. Furchgott, Louis J. Ignarro, Ferid Murad: nitric oxide as a signalling molecule in the cardiovascular system. Nobel Prizes in revealing the Metabolism of living matter (5) • 1999, Gunter Blobel: protein localization. • 2000, Arvid Carlsson, Paul Greengard, Eric R. Kandel: signal transduction in the nervous system. • 2001, Leland H. Hartwell, Tim Hunt, Sir Paul Nurse: regulators of the cell cycle. • 2002, Sydney Brenner, H. Robert Horvitz, John E. Sulston: regulation of organ development and programmed cell death. • 2004, Aaron Ciechanover, Avram Hershko, Irwin Rose: ubiquitin-mediated protein degradation. Major aspects that will be covered in Biochemistry II • General principles for bioenergetics. • Oxidative degradation of fuels (glycolysis, boxidation, a-ketoacid oxidation, citric acid cycle), generating NADH, FADH2, ATP, and CO2. • Oxidation of NADH and FADH2 by O2 and generation of ATP and H2O (respiratory chains, ATP synthase). • Biosynthesis of carbohydrates (including photosynthsis), fatty acids, amino acids, and nucleotides. • Metabolites, chemical reactions, enzymes, regulations, with wide applications in medicine, agriculture, and biotechnology. How to study metabolism • Compare and relate (interconnect) the chemical reactions (Where are you in the metabolism network?) • Try to contemplate on the ways the living organisms used to achieve a balanced and dynamic steady state (How could the multilayered regulation cooperate so effectively?). • Understand the classical experiments and thoughts that led to the revelation of the knowledge described (Does He/she deserve the Nobel Prize?). • Be aware of the nature of the data (Could this observations from in vitro studies be extended to what happens in vivo?). • Understand the aspects that need further studies (Do I still have a chance to win a Nobel Prize?). Enjoy Biochemistry II: a course that will allow you to learn what life is really all about. Scoring policies for this course • Tests (attendance): 10%; • Critical reading of a research paper (one paper for each two students): 15%; • Final Exam: 75%. Date Chapter Lecturer Sept. 12 Over view of metabolism and Chapter 13: Principles of Bioenergetics Dr. Zengyi Chang Sept. 19 Chapter 14 Glycolysis & and Pentose phosphate pathway Dr. Zengyi Chang Sept. 26 Chapter 16 The Citric Acid Cycle Dr. Zengyi Chang Oct. 10 Chapter 17 Fatty Acid Catabolism Dr. Yongmei Qin Oct. 17 Chapter 18 Amino Acid Oxidation & Production of Urea Dr. Yongmei Qin Oct. 24 Chapter 18 Amino Acid Oxidation & Production of Urea Dr. Yongmei Qin Oct. 31 Chapter 19 Oxidative phosphorylation and photophosphorylation Dr. Zengyi Chang Nov. 7 Chapter 19 Oxidative phosphorylation and photophosphorylation Dr. Zengyi Chang Nov. 14 Chapter 14 GLuconeogenesis Chapter 15 Principles of Metabolic regulation: Glucose and GLycogen Dr. Yongmei Qin Nov. 21 Chapter 20 Carbohydrate Biosynthesis in plants Dr. Yongmei Qin Nov. 28 Chapter 21 Lipid biosynthesis Dr. Yongmei Qin Dec. 5 Chapter 21 Lipid biosynthesis Dr. Yongmei Qin Dec. 12 Chapter 22 Biosynthesis of amino acids, nucleotides and related molecules Dr. Zengyi Chang Dec. 19 Dec 26 Chapter 22 Biosynthesis of amino acids, nucleotides and related molecules Chapter 23 Integration and hormonal regulation of mammalian metabolism Dr. Zengyi Chang Dr. Zengyi Chang