Estrogen Alters Ischemia-Reperfusion Injury
advertisement
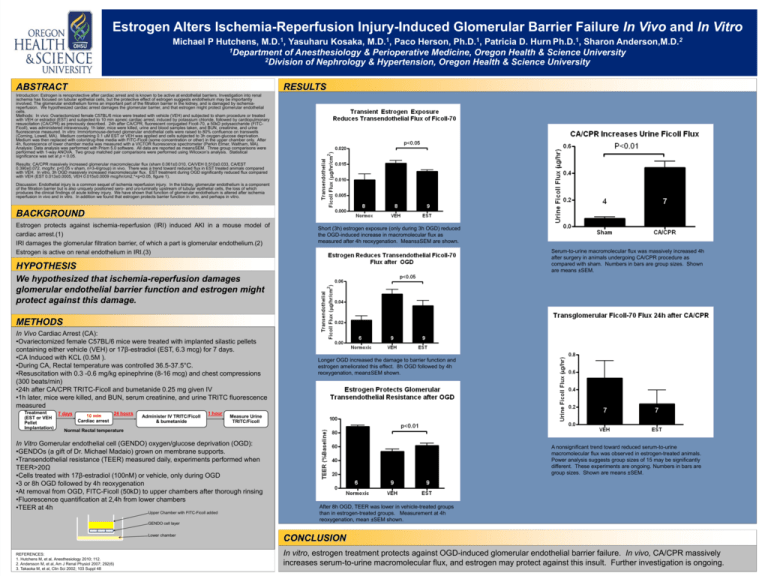
Estrogen Alters Ischemia-Reperfusion Injury-Induced Glomerular Barrier Failure In Vivo and In Vitro Michael P Hutchens, M.D.1, Yasuharu Kosaka, M.D.1, Paco Herson, Ph.D.1, Patricia D. Hurn Ph.D.1, Sharon Anderson,M.D.2 1Department of Anesthesiology & Perioperative Medicine, Oregon Health & Science University 2Division of Nephrology & Hypertension, Oregon Health & Science University ABSTRACT RESULTS Introduction: Estrogen is renoprotective after cardiac arrest and is known to be active at endothelial barriers. Investigation into renal ischemia has focused on tubular epithelial cells, but the protective effect of estrogen suggests endothelium may be importantly involved. The glomerular endothelium forms an important part of the filtration barrier in the kidney, and is damaged by ischemiareperfusion. We hypothesized cardiac arrest damages the glomerular barrier, and that estrogen might protect glomerular endothelial cells. Methods: In vivo: Ovariectomized female C57BL/6 mice were treated with vehicle (VEH) and subjected to sham procedure or treated with VEH or estradiol (EST) and subjected to 10 min apneic cardiac arrest, induced by potassium chloride, followed by cardiopulmonary resuscitation (CA/CPR) as previously described. 24h after CA/CPR, fluorescent conjugated Ficoll-70, a 50kD polysaccharide (FITCFicoll), was administered intravenously. 1h later, mice were killed, urine and blood samples taken, and BUN, creatinine, and urine fluorescence measured. In vitro: Immortomouse-derived glomerular endothelial cells were raised to 80% confluence on transwells (Corning, Lowell, MA). Medium containing 0.1 uM EST or VEH was applied and cells subjected to 3h oxygen-glucose deprivation. Medium was then replaced with color/drug-free media with FITC-Ficoll (some concentration or other) in the upper chamber only. After 4h, fluorescence of lower chamber media was measured with a VICTOR fluorescence spectrometer (Perkin Elmer, Waltham, MA). Analysis: Data analysis was performed with Prism 5.0 software. All data are reported as mean±SEM. Three group comparisons were performed with 1-way ANOVA. Two group matched pair comparisons were performed using Wilcoxon’s analysis. Statistical significance was set at p < 0.05. Results: CA/CPR massively increased glomerular macromolecular flux (sham 0.061±0.010, CA/VEH 0.510±0.033, CA/EST 0.390±0.072, mcg/hr, p<0.05 v sham, n=3-4/group) in vivo. There was a trend toward reduced flux in EST treated animals compared with VEH. In vitro, 3h OGD massively increased macromolecular flux. EST treatment during OGD significantly reduced flux compared with VEH (EST 0.013±0.0005, VEH 0.015±0.0009 mcg/hr/cm2,*=p<0.05, figure 1). Discussion: Endothelial injury is a common sequel of ischemia reperfusion injury. In the kidney, glomerular endothelium is a component of the filtration barrier but is also uniquely positioned sero- and uro-luminally upstream of tubular epithelial cells, the loss of which produces the clinical findings of acute kidney injury. We have shown that function of glomerular endothelium is altered after ischemia reperfusion in vivo and in vitro. In addition we found that estrogen protects barrier function in vitro, and perhaps in vitro. BACKGROUND Estrogen protects against ischemia-reperfusion (IRI) induced AKI in a mouse model of cardiac arrest.(1) IRI damages the glomerular filtration barrier, of which a part is glomerular endothelium.(2) Estrogen is active on renal endothelium in IRI.(3) Short (3h) estrogen exposure (only during 3h OGD) reduced the OGD-induced increase in macromolecular flux as measured after 4h reoxygenation. Means±SEM are shown. Serum-to-urine macromolecular flux was massively increased 4h after surgery in animals undergoing CA/CPR procedure as compared with sham. Numbers in bars are group sizes. Shown are means ±SEM. HYPOTHESIS We hypothesized that ischemia-reperfusion damages glomerular endothelial barrier function and estrogen might protect against this damage. METHODS In Vivo Cardiac Arrest (CA): •Ovariectomized female C57BL/6 mice were treated with implanted silastic pellets containing either vehicle (VEH) or 17β-estradiol (EST, 6.3 mcg) for 7 days. •CA Induced with KCL (0.5M ). •During CA, Rectal temperature was controlled 36.5-37.5°C. •Resuscitation with 0.3 -0.6 mg/kg epinephrine (8-16 mcg) and chest compressions (300 beats/min) •24h after CA/CPR TRITC-Ficoll and bumetanide 0.25 mg given IV •1h later, mice were killed, and BUN, serum creatinine, and urine TRITC fluorescence measured Treatment 24 hours 7 days 10 min (EST or VEH Cardiac arrest Pellet Implantation) Normal Rectal temperature Administer IV TRITC/Ficoll & bumetanide 1 hour Measure Urine TRITC/Ficoll In Vitro Gomerular endothelial cell (GENDO) oxygen/glucose deprivation (OGD): •GENDOs (a gift of Dr. Michael Madaio) grown on membrane supports. •Transendothelial resistance (TEER) measured daily, experiments performed when TEER>20Ω •Cells treated with 17β-estradiol (100nM) or vehicle, only during OGD •3 or 8h OGD followed by 4h reoxygenation •At removal from OGD, FITC-Ficoll (50kD) to upper chambers after thorough rinsing •Fluorescence quantification at 2,4h from lower chambers •TEER at 4h Upper Chamber with FITC-Ficoll added GENDO cell layer Lower chamber REFERENCES: 1. Hutchens M, et al, Anesthesiology 2010; 112. 2. Andersson M, et al, Am J Renal Physiol 2007; 292(6) 3. Takaoka M, et al, Clin Sci 2002; 103 Suppl 48 Longer OGD increased the damage to barrier function and estrogen ameliorated this effect. 8h OGD followed by 4h reoxygenation, mean±SEM shown. A nonsignificant trend toward reduced serum-to-urine macromolecular flux was observed in estrogen-treated animals. Power analysis suggests group sizes of 15 may be significantly different. These experiments are ongoing. Numbers in bars are group sizes. Shown are means ±SEM. After 8h OGD, TEER was lower in vehicle-treated groups than in estrogen-treated groups. Measurement at 4h reoxygenation, mean ±SEM shown. CONCLUSION In vitro, estrogen treatment protects against OGD-induced glomerular endothelial barrier failure. In vivo, CA/CPR massively increases serum-to-urine macromolecular flux, and estrogen may protect against this insult. Further investigation is ongoing.










