docx - Mini
advertisement

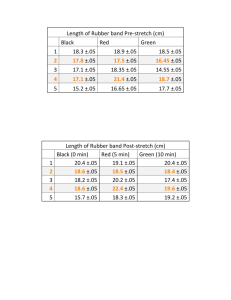
Mini-Lab 3-04: Banding Together Purpose: Just like chemical reactions, physical processes can be either endothermic or exothermic. Think about this: ΔG = ΔH - TΔS Where G is free energy, H is enthalpy and S is entropy and T is temperature in absolute Kelvins. For a process or reaction to be spontaneous, ΔG must be negative Safety 1) 2) 3) 4) Don’t eat or drink anything in the lab. Always wear eye protection. Wear protective clothing (lab coats, etc.). Don’t play around – treat the lab with respect. Questions 1) Hold a rubber band between two fingers and rapidly stretch it outwards. a. Quickly hold it to your forehead or cheek. What do you observe? b. Stretch it again and then let it contract. Quickly hold it to your forehead or cheek again. What do you observe? 2) Here is a “reaction equation” for what you just did: Relaxed rubber band stretched rubber band a. Where does “heat” appear in this equation? Write it on the appropriate side of the arrow. 3) In which direction is this reaction spontaneous (relaxed stretched OR stretched relaxed)? 4) For the spontaneous process: a. Draw a picture of what you think the molecules in the rubber band look like when it is stretched, and then when it is relaxed. b. What are the signs of ΔG, ΔH and ΔS? c. When the rubber band contracts, the molecules are getting ______________________ and ____________________. WAIT! Do not write down an answer to the Final question until your Instructor tells you to. 5) FINAL: Based on your observations, will the rubber band stretch out if you heat it up? Instructor’s Page 3-04: Thermodynamics and rubber bands? Source: USAFA Demo 12.5, and SCALE-UP Concepts: endo- and exo-thermic physical processes, equilibrium, entropy Materials: rubber bands (thick ones work the best), one hair dryer or heat gun (for the Instructor) Hints: This is very simple and can also be run as a contest – you can split the class up into three groups: those who think it will expand, those who think it will contract, and those who think it will not change. After they make their prediction, heat a rubber band with a hair dryer or heat-gun (better). When heated, the rubber band will basically stay unchanged, but if you look closely, you will see that it contracts along the short axis of the band. When you heat the rubber band, hold it with some tongs so that it hangs down, which makes it even more obvious that it does NOT become any more stretched out and droopy as it gets hot. Since the change in heat for the spontaneous process (contracting) is positive (endothermic – it feels cold), then the entropy change must also be positive and must be larger than the enthalpy change, since that is the only way for this process to achieve a negative change in free energy (i.e. – be spontaneous). This means that when the rubber band contracts, the molecules must be getting more energetic and LESS ordered (entropy is increasing). When you stretch the rubber band, the coiled molecules are stretched out and aligned, decreasing the amount of disorder (entropy) in the band. Another way to think of entropy is as “opportunity to achieve different orientations or energy states. When the molecules in the band are stretched, they are locked into more rigid orientations (think of a bunch of bungee cords that have been stretched out). When the band relaxes, the molecules have more opportunity to move about and coil around each other – entropy (opportunity) has increased. Since enthalpy is positive and entropy is also large and positive for the contraction process, then, no matter how high the temperature gets (as long as the rubber band does not disintegrate or burst into flame), there is no possible way for the expansion process to become spontaneous. In fact, the only way to have a chance of making expansion of the rubber band spontaneous would be to cool it down. Even thought the students should be able to reason this out based on the derived signs of enthalpy and entropy, many of them will stick to their mental model that hot things should stretch out. Discussion ideas: Be sure to discuss the signs of enthalpy and entropy (refer to above explanation) so everyone is clear on what they are. You might want to discuss how difficult it is to let go of a preconceived notion, even in the light of new information. In fact, this is why it is so difficult to teach science – it often takes many examples of overwhelming evidence to sway people from their old ideas.