60 and < 90 cc/min
advertisement

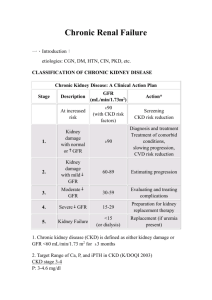
HIV and Renal Health Dr. Patrice Junod Clinique médicale l’Actuel This activity is supported by an educational grant from: Program Development Principle Content Development Anita Rachlis Ali Zahirieh David Fletcher Content Contributors Gord Arbess Brian Conway Marianne Harris Patrice Junod Graham Smith Alice Tseng Consultant Linda Robinson Jean-Guy Baril Chris Fraser Christine Hughes Marek Smieja Rachel Therrien Sharon Walmsley Mélanie Hamel Conflict of Interest Declaration This program was developed with consultants through an educational grant from Janssen Inc. The faculty members received financial compensation for developing & presenting this program. Faculty Disclosures • • • • • Abbvie Gilead Janssen Merck ViiV Objectives • Discuss factors that can impact renal health in HIV patients • List which renal lab tests are the most clinically relevant and how often they should be performed • Present a practical tool for the management of declining renal function • Apply these learnings using interactive patient case examples Background: HIV and the Kydney Renal Disease in HIV Positive Patients • Kidney disease is an important complication of HIV infection in the era of antiretroviral therapy1 • In a retrospective study of 487 consecutive HIV positive patients with normal renal function, the initial prevalence of CKD was 2%2 – After 5 years of follow-up, 6% had progressed to CKD – Older age was a multivariate predictor of CKD for this cohort 1 2 Gupta SK, et al. Clinical Infectious Disease 2005;40:1559-1585. Gupta SK, et al. Clinical Nephrology 2004;61:1-6. Kidney Disease in HIV Positive Patients • The spectrum of kidney disease in HIV includes: – HIV-associated nephropathy – Immune complex kidney disease – Medication nephrotoxicity – Kidney disease related to co-morbid conditions • Diabetes, hypertension, and hepatitis virus co-infection Wyatt CM. AJM 2007;120:488-49. Risk Factors for Kidney Disease in the HIV Positive Population Ethnicity Family History Age HIV = Modifiable = Nonmodifiable CKD Risk Nephrotoxic medication Hypertension Diabetes Hepatitis C Gupta SK, et al. Clinical Infectious Disease 2005;40:1559-1585. Chronic Kidney Disease in HIV • Prevalence 3-15% • Race and other genetic factors • Hypertension • Diabetes mellitus • Hepatitis C virus infection • Decreased CD4 cell count • Increased viral load • Nephrotoxic Drugs Medications and Renal Disease Prerenal ACE-I ARBs Direct Renin Inhibitors Amphotericin NSAIDS Cyclosporine Diuretics Interferon Tubular Injury Cidofovir Adefovir Tenofovir Didanosine Lamivudine Stavudine Aminoglycosides Amphotericin Cocaine Foscarnet Pentamidine Ketamin Allergic Interstitial Nephritis Abacavir Indinavir Ritonavir Atazanavir Acyclovir Cephalosporins Penicillins Ciprofloxacin TMP/SMX Rifampin NSAIDs Proton Pump Inhibitors Thrombotic Microangiopathy Indinavir Cocaine Cyclosporine Valacyclovir TMP/SMX: trimethoprim and sulfamethoxazole Adapted from: Guo X, Nzerue C. Cleve Clin J Med 2002;69:289-312. Obstructive Indinavir Atazanavir Acyclovir Foscarnet Sulfadiazine TMP/SMX HIV & The Kidney: Summary • Acute Kidney Injury (AKI) is more common in individuals with HIV infection • Chronic Kidney Disease (CKD) is more common in individuals with HIV infection • Proteinuria is more common in individuals with HIV infection • Proximal tubular dysfunction is more common in individuals with HIV infection Classification of CKD Stage Description GFR (mL/min/1.73m2) I Urinary and/or Structural Abnormality > 89 II Urinary and/or Structural Abnormality 60 - 89 IIIa Mild GFR decline 45 - 59 IIIb Moderate GFR decline 30 - 44 IV Severe GFR decline 15 - 29 V Kidney Failure < 15 ESRD Requiring Renal Replacement Therapy Levey A. KI 2010;80: 17. Three Important Measures • Glomerular Filtration Rate (GFR) • Proteinuria • Proximal Tubular Function Three Important Measures • Glomerular Filtration Rate (GFR) • Proteinuria • Proximal Tubular Function How Do We Measure GFR? • Gold standard: – inulin clearance – iothalamate clearance – Iohexol • “Practical” – serum creatinine – 24-hr urine collection for creatinine clearance (cumbersome!) – equations, equations, equations Renal Function Measurement • Serum creatinine – Metabolism of creatine in skeletal muscle and from dietary meat intake – Production tied to muscle mass • Age, weight, sex, amputations, corticosteroid use – Modestly influenced by diet – Filtered by glomerulus and secreted by proximal tubule • Proportionally increased secretion with reduced GFR – Creatinine may not increase until up to 50% of GFR is lost • Secretion inhibited by drugs including cimetidine, trimethoprim, dapsone, cobicistat – Large intra-person and intra-laboratory variation • Intra-person variation 7−20% • Poor intra-laboratory calibration particularly affecting higher GFRs Krop JS, et al. Arch Intern Med 1999;159:1777-1783. Coresh J, et al. Am J Kidney Dis 2002;39:920-929. Serum Creatinine 110 μmol/L Serum Creatinine 110 μmol/L 40 ml/min 140 ml/min The same serum creatinine represents very different GFRs in these two individuals Cockcroft-Gault Equation CrCl = weight x (140 – age) / (serum Cr x 49)* •Estimates CrCl (not GFR) •Derived from a study of 249 white Canadian hospitalized veterans who had 2 similar 24-hr urine CrCl measurements •Validated for renal dosing of drugs * X 0.8 if female MDRD Equation • MDRD GFR (mL/min/1.73m2) = 175 x [serum creatinine(µmol/L)/88.4]-1.154 x (Age) -0.203 x (0.742 if female) x (1.21 if African American) • Estimates Glomerular Filtration Rate • Derived from 1070 individuals with advanced chronic kidney disease • 60% male, 88% white, 6% DM CKD-EPI • Newest Equation of the three • Non-linear based equation • More accurate in estimating GFR in those with mild CKD Levey et al. Ann Intern Med 2009;150: 604-612. Three Important Measures • Glomerular Filtration Rate (GFR) • Proteinuria • Proximal Tubular Function Quantifying Proteinuria • Normal – < 150 mg/day of proteinuria – < 30 mg/day of albuminuria • Quantification strategies: – Dipstick • Measure ONLY albumin at a CONCENTRATION > 300 mg/L – 24-hr urine collection • Helpful if patient performs a ‘complete’ collection – Spot urine albumin:creatinine (or protein:creatinine) • Can increase sensitivity for detecting proteinuria in a convenient fashion Practical Point • A typical man produces roughly 15 mmol of creatinine/day • A typical woman produces roughly 10 mmol of creatinine/day • The protein:creatinine (PCR) or albumin:creatinine (ACR) tell you how much protein/albumin is present in the urine per mmol of Cr • Thus multiplying the ACR by 10 in woman and by 15 in men will give you an estimate of that individual’s 24hr excretion of albumin (the exact same applies to PCR) Implications of Proteinuria • A marker of increased risk of CV events • Increased risk of CKD progression – (notably when > 1g/day protein or 200mg/day albumin) Three Important Measures • Glomerular Filtration Rate (GFR) • Proteinuria • Proximal Tubular Function Tubular Functions • “Reabsorption” – Water – Electrolytes – Bicarbonate – Glucose – Filtered proteins • Secretion – Organic Anions/Cations – Drugs – Metabolic Byproducts • Creatinine Ernst M, Moser M. N Engl J Med 2009;361:2153-2164. Proximal Tubular Function • Protein Reabsorption • Phosphate Reabsorption • Glucose Reabsorption • Amino Acid Reabsorption • Creatinine Secretion • Bicarbonate “reabsorption” Ernst M, Moser M. N Engl J Med 2009;361:2153-2164. “What Are You Looking For?” • Some Evidence of Proximal Tubular Injury – Urine: • glucosuria in absence of diabetes • Non-albumin based proteinuria – measure both albuminuria & proteinuria – high urinary β2-microglobulin excretion • Evidence of ATN (hemegranular casts) – Serum: • non-anion gap metabolic acidosis, creatinine rise • Hypophosphatemia & high urinary phosphate excretion – Calculate the Fractional Excretion of Phosphate* – (Urinary PO4/Ur Cr) / (Serum PO4/Serum Cr) – Abnomal = greater than 10% in setting of hypophosphatemia Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy • Aquitaine Cohort • 399 patients in a cross sectional analysis • Overall prevalence of PRTD was high at 6.5 % • 29.6 % stage 1 or 2 kidney disease • 5.3 % stage 3 to 5 kidney disease F-A Dauchy et al. Kidney International 2011;80:302-309. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy • Multivariate Analysis showed significant independent associations • Age (OR 1.28 per 5 year increase) • TDF (OR 1.23 per year) • ATZ (OR 1.28) • Primary tubular abnormalities can be missed even when severe and can lead to decline in GFR • Early screening is necessary to avoid them F-A Dauchy et al. Kidney International 2011;80:302-309. Guidelines IDSA Guidelines: Evaluating and Monitoring CKD in HIV • All patients at the time of HIV diagnosis should be assessed for existing kidney disease – Calculated estimate of renal function and – Screening for proteinuria • Dipstick, protein/creat ratio or albumin/creat ratio? • If there is no evidence of kidney disease at initial evaluation, patients at high risk for the development of proteinuric renal disease should undergo annual screening – African American persons – CD4+ cell counts <200 mL or HIV RNA levels >4000 copies/mL – Diabetes mellitus – Hypertension – Hepatitis C virus coinfection • Patients without risk factors for kidney disease should be followed clinically and reassessed based on the occurrence of signs and symptoms or as clinical events dictate Gupta SK et al. Clin Infect Dis 2005;40:1559-1585. IDSA Initial Evaluation Recommendations • Obtain baseline GFR: – All patients at the time of HIV diagnosis should be assessed for existing kidney disease with a screening urinalysis for proteinuria and a calculated estimate of renal function • Annual screening: – If there is no evidence of proteinuria at initial evaluation, patients at high risk for the development of proteinuric renal disease should undergo annual screening – Renal function should be estimated on a yearly basis to assess for changes over time • When to consider a nephrology consult: – Additional evaluations and referral to a nephrologist are recommended for patients with proteinuria of grade ≥1+ by dipstick analysis or GFR<60 mL/min per 1.73m2 Gupta SK, et al. Clinical Infectious Disease 2005;40:1559-1585. DHHS Recommendations Table 3. Laboratory Monitoring Schedule for Patients Before and After Initiation of Antiretroviral Therapy Entry info care Follow-up before ART ART Initiation or modification Follow-up 2-8 weeks postART initiation or modification Every 3-6 months ALT, AST, T bilirubin Every 6-12 months CBC with differential Every 3-6 months If on ZDV Fasting lipid profile If normal annually Consider 4-8 weeks after starting new ART regimen that affects lipids Fasting glucose or hemoglobin A1C If normal annually Urinalysis Pregnancy test If startting EFV Every 6 months If abnormal at last measurement If abnormal at last measurement Every 12 months If abnormal at last measurem ent If abnormal at last measurement If on TDF Treatme nt failure Clinically indicated Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/ContentFiles/Adultand Emerging Evidence • Kidney Stones • Chronic Kidney Disease (CKD) Renal Stones • Renal stones are risk factor for chronic kidney disease (CKD) • Urolithiasis well-known side effect of indinavir – Considered to be drug crystallization in urine • Urolithiasis also associated with atazanavir – Probably similar etiology Hamada et al. Clin Infect Dis, 2012 ATV & Renal Stones: Hamada et al. • Cohort analysis of 1240 patients – ATV/r (n=465) or other protease inhibitors (n=775) • Renal stones developed in 31 patients on ATV/r (6.7%) and 4 patients (0.52%) on other PIs – Risk was 10 times higher in ATV/r group • Patients on ATV/r had lower eGFR – Lower eGFR associated with renal stones Hamada et al. Clin Infect Dis, 2012 ATV & Renal Stones: Rockwood et al. ATV (n = 1,206) EFV / DRV / LPV combined cohort (n=4,449) 24 24 Prevalence of kidney stones per 1,000 patients (95% CI) 20 (13 - 30) 5.4 (3.2 – 7.6) < 0.001 Event rate per 1,000 pt-yrs of exposure, n (95% CI) 7.3 (4.7 - 10.8) 1.9 (1.2 - 2.8) < 0.001 No. of patients with kidney stones p value • Event rate remained significantly higher in the ATV cohort after adjusting for prior ATV and IDV exposure • ATV/r patients who developed renal stones had significantly higher bilirubin levels vs. ATV/r patients who did not develop stones • At study baseline, 42% of ATV/r patients who developed renal stones had chronic renal impairment vs. 4.5% of ATV/r patients who did not develop stones Rockwood N, et al. 17e conférence annuelle de la BHIVA, Bournemouth, 2011, résumé O4. Renal Impairment PI’s vs EFV Hazard ratio* (95% CI) p value LPV/r 1.69 (1.1 - 2.6) 0.017 ATV/r 1.52 (1.14 - 2.03) 0.004 DRV/r 1.31 (0.94 à 1.81) 0.108 EFV 1.00 *Adjusted for gender, age at start of HAART, ethnicity, baseline eGFR, baseline CD4 cell count, baseline viral load, HBsAg, prior exposure to TDF and IDV and total duration of TDF exposure Au cours des 12 premiers mois, 49 % des sujets ayant développé une insuffisance rénale s’étaient rétablis (TFGe > 60 ml/min/1,73 m2). Rockwood et al., J Antivir Antiretrovir 2012,N, 4:2et al. J Antivir Antiretrovir 2012;4: 21-25. Rockwood ACTG 5202: Creatinine Clearance Median Change in Calculated Creatinine from Baseline (mL/min) 10 Median Creatinine Clearance: ATV/r vs. EFV 8 6 ** * p = 0,001 p/r at ATV/r ** p < 0,001 p/r at ATV/r * 4 Week 4848 Week Week 9696 Week 2 ATV/r 0 EFV ATV/r EFV -2 +ABC/3TC -4 n 338 287 377 330 +TDF/FTC 360 327 Daar et al. Ann Intern Med, 2011 394 352 Chronic Kidney Disease & ARV Exposure Incidence of CKD with Each Additional Year of Exposure Annual Increased Risk Medication Atazanavir + Tenofovir 41 % Atazanavir 22 % Tenofovir 16 % Indinavir 11 % Lopinavir/r 8% Mean follow up was 3.7 years N = 6,843 Adapté de Mocroft et al. AIDS, 2010 ARVs & Renal Impairment: The D:A:D Cohort • Cohort of 49,734 • First analysis to focus on patients with normal renal function at baseline (n=22,603) – eGFR > 90 ml/min/1.73m2 • Followed to confirmed: – eGFR < 70 ml/min/1.73m2 – Or eGFR < 60 ml/min/1.73m2 – Or last available eGFR Ryom et al. Présentation d’affiche, CROI, 2012 D:A:D Cohort: Results • N=22,603 • 4.5 year follow up • 468 (2.1%) patients progressed to eGFR < 70 – • 131 (0.6%) patients progressed to CKD – • incidence rate 4.78/1000 patient years incidence rate 1.33/1000 patient years Equals an annual decline of at least 4-5 ml/min CKD=Chronic Kidney Disease Ryom et al. Présentation d’affiche, CROI, 2012 ARVs Exposure Rates of ceGFR <70 from eGFR > 90 (adjusted analysis) Ryom et al. Poster presentation, CROI, 2012 Canadian Observational Cohort (CANOC) Collaboration Time to Impaired eGFR Medication Adjusted Hazard Ratio (95% CI) P Value Non PI 1.00 Tenofovir 1.16 0.177 Lopinavir 1.32 0.024 Atazanavir 1.46 < 0.001 N = 965 Adapté de Hosein et al. Présentation d’affiche, IAS, 2011 EuroSIDA Study: Risk for Chronic Kidney Disease • Analysis of patients with ≥ 3 creatinine measurements + body weight – 6,842 patients with 21,482 person-years of follow-up • Definition of CKD (eGFR by Cockcroft-Gault) – If baseline eGFR ≥60 mL/min/1.73 m2, fall to <60 – If baseline eGFR <60 mL/min/1.73 m2, fall by 25% • 225 (3.3%) progressed to CKD Cumulative Exposure to ARVs and Risk of CKD Univariable Multivariable IRR/year 95% CI P-value IRR/year 95% CI P-value Tenofovir 1.32 1.21-1.41 <0.0001 1.16 1.06-1.25 <0.0001 Indinavir 1.18 1.13-1.24 <0.0001 1.12 1.06-1.18 <0.0001 Atazanavir 1.48 1.35-1.62 <0.0001 1.21 1.09-1.34 0.0003 Lopinavir/r 1.15 1.07-1.23 <0.0001 1.08 1.01-1.16 0.030 Risk factors for CKD on TDF: age, HTN, HCV, lower eGFR, lower CD4+ count Kirk O, et al. 17th CROI; San Francisco, CA; February 16-19, 2010. Abst. 107LB. EuroSIDA STUDY: Crude Incidence Rate of CKD and Increasing Exposure to ARVs Incidence per 100 PYFU (95% CI) N with CKD 10 86 21 34 29 55 Tenofovir 67 31 35 25 67 Indinavir 127 20 19 11 48 Atazanavir 143 23 20 18 21 Lopinavir/r 1 .01 Not 0-1 1-2 2-3 started >3 Not 0-1 1-2 2-3 started >3 Not 0-1 1-2 2-3 started >3 Not 0-1 1-2 2-3 started >3 Years of Exposure to ARV CKD, confirmed (persisting for >3 months) decrease in eGFR ≤60 mL/min/1.73m2 if eGFR at baseline >60 mL/min/1.73m2 or confirmed 25% decrease in eGFR if baseline eGFR≤60 mL/min/1.73m2 Kirk, CROI 2010; 107LB. Algorithm 1- Algorithm Nephropathy Advisory Committee on the clinical management of people living with HIV 2- HIV and Renal Health – Management tool National Development Committee – Supported by Janssen PERIODIC HEALTH EXAMINATION OF ADULTS LIVING WITH HIV (HUMAN IMMUNODEFICIENCY VIRUS) − Nephropathy − Advisory Committee on the Clinical Management of Persons Living with HIV Screening for Kidney Problems Advisory Committee on the Clinical Management of Persons Living with HIV Screening schedule based on risk factors for kidney disease (EACS 2011) Untreated HIV+ patients Assessment of risk factors for CKD* Urinalysis or urine dipstick eGFR Phosphorus Treated HIV+ patients Without TDF With TDF Annual Annual 6–12 months Annual Annual 6 months if GFR < 60 3-6 months 6-12 months 3-6 months 3-6 months As needed As needed Optional 3-6 months * Risk factors for CKD: Diabetes, hypertension, CVD, viral hepatitis, concomitant nephrotoxic drugs, family history of CKD, black African ethnicity GFR using CKD-EPI or MDRD > 60 and < 90 cc/min < 60 cc/min* Increase in Cr > 20% for > 3 months** Repeat CKD-EPI or MDRD calculation < 30 cc/min* Glucose+ Protein+ HypoPO4 ACR and MAU CaPO4 Renal ultrasound Refer to algorithms (next pages) GFR < 90 GFR > 90 Follow up every 3 months Regular follow-up Refer to proteinuria algorithm (next page) Referral to nephrologist or internist * If GFR < 50 cc/min: consider adjusting the dose of certain ARV and concomitant medications ** Test for tubulopathy if GFR declines > 10 cc/min while on tenofovir GFR using CKD-EPI or MDRD > 60 and < 90 cc/min < 60 cc/min* Increase in Cr > 20% for > 3 months** Repeat CKD-EPI or MDRD calculation < 30 cc/min* Glucose+ Protein+ HypoPO4 ACR and MAU CaPO4 Renal ultrasound Refer to algorithms (next pages) GFR < 90 GFR > 90 Follow up every 3 months Regular follow-up Refer to proteinuria algorithm (next page) Referral to nephrologist or internist * If GFR < 50 cc/min: consider adjusting the dose of certain ARV and concomitant medications ** Test for tubulopathy if GFR declines > 10 cc/min while on tenofovir > 60 and < 90 cc/min Increase in Cr > 20% for > 3 months** Glucose+ Protein+ HypoPO4 Repeat CKD-EPI or MDRD Refer to algorithms (next pages) calculation GFR using CKD-EPI or MDRD * If GFR < 50 cc/min: consider adjusting the dose of certain ARV and concomitant medications GFR < 90 GFR > 90 ** Test for tubulopathy if GFR declines > 10 cc/min while on tenofovir Follow up every 3 months Regular follow-up GFR using CKD-EPI or MDRD > 60 and < 90 cc/min < 60 cc/min* Increase in Cr > 20% for > 3 months** Repeat CKD-EPI or MDRD calculation < 30 cc/min* Glucose+ Protein+ HypoPO4 ACR and MAU CaPO4 Renal ultrasound Refer to algorithms (next pages) GFR < 90 GFR > 90 Follow up every 3 months Regular follow-up Refer to proteinuria algorithm (next page) Referral to nephrologist or internist * If GFR < 50 cc/min: consider adjusting the dose of certain ARV and concomitant medications ** Test for tubulopathy if GFR declines > 10 cc/min while on tenofovir GFR using CKD-EPI or MDRD < 60 cc/min* ACR and MAU Refer to proteinuria algorithm (next page) < 30 cc/min* CaPO4 Renal ultrasound Referral to nephrologist or internist * If GFR < 50 cc/min: consider adjusting the dose of certain ARV and concomitant medications ** Test for tubulopathy if GFR declines > 10 cc/min while on tenofovir Urinalysis or urine dipstick Glucose > 0 Protein ≥ 1 + or 0.25 g/L Fasting glucose + Rule out diabetes Repeat at next appt. Glycosuri a DB + Glycosuri a DB – Protein ≥ 1+ or 0.25 g/L Protein < 1+ or 0.25 g/L DB follow-up Repeat 1x ACR and MAU Normal Glycosuri a DB – Referral to nephrologist or internist ACR > 0.05 g/mmol or MAU > 2.1 mg/mmol or hematuria (> 2 RBC/HPF) - Renal ultrasound - Ascertain the risk factors - Referral to nephrologist or internist, or to urologist for isolated hematuria ACR ≤ 0.05 g/mmol and MAU < 2.1 mg/mmol Normal Urinalysis or urine dipstick Glucose > 0 Protein ≥ 1 + or 0.25 g/L Fasting glucose + Rule out diabetes Repeat at next appt. Glycosuri a DB + Glycosuri a DB – Protein ≥ 1+ or 0.25 g/L Protein < 1+ or 0.25 g/L DB follow-up Repeat 1x ACR and MAU Normal Glycosuri a DB – Referral to nephrologist or internist ACR > 0.05 g/mmol or MAU > 2.1 mg/mmol or hematuria (> 2 RBC/HPF) - Renal ultrasound - Ascertain the risk factors - Referral to nephrologist or internist, or to urologist for isolated hematuria ACR ≤ 0.05 g/mmol and MAU < 2.1 mg/mmol Normal Urinalysis or urine dipstick Glucose > 0 Fasting glucose + Rule out diabetes Glycosuri a DB + Glycosuri a DB – DB follow-up Repeat 1x Glycosuri a DB – Referral to nephrologist or internist Urinalysis or urine dipstick Glucose > 0 Protein ≥ 1 + or 0.25 g/L Fasting glucose + Rule out diabetes Repeat at next appt. Glycosuri a DB + Glycosuri a DB – Protein ≥ 1+ or 0.25 g/L Protein < 1+ or 0.25 g/L DB follow-up Repeat 1x ACR and MAU Normal Glycosuri a DB – Referral to nephrologist or internist ACR > 0.05 g/mmol or MAU > 2.1 mg/mmol or hematuria (> 2 RBC/HPF) - Renal ultrasound - Ascertain the risk factors - Referral to nephrologist or internist, or to urologist for isolated hematuria ACR ≤ 0.05 g/mmol and MAU < 2.1 mg/mmol Normal Urinalysis or urine dipstick Protein ≥ 1 + or 0.25 g/L Repeat at next appt. Protein ≥ 1+ or 0.25 g/L Protein < 1+ or 0.25 g/L ACR and MAU Normal ACR > 0.05 g/mmol or MAU > 2.1 mg/mmol or hematuria (> 2 RBC/HPF) - Renal ultrasound - Ascertain the risk factors - Referral to nephrologist or internist, or to urologist for isolated hematuria ACR ≤ 0.05 g/mmol and MAU < 2.1 mg/mmol Normal Serum phosphorus 0.65 – normal level Repeat in 3 months 0.32 – 0.65 mmol/L Repeat in 1 month < 0.32 mmol/L < normal levels Treat immediately Referral to nephrologist Repeat and if < normal levels 25-OH Vit D < 50: deficiency < 75: insufficiency Vit D Rx > 75 Normal PTH assay Abnorma l Referral to nephrologist or internist Normal Urinary fractional excretion of phosphorus if available (if > 20% or > 10% and hypophosphatemia: referral to a specialist Albumincorrected Ca Abnorma l Referral to nephrologist or internist Normal Serum phosphorus 0.65 – normal level Repeat in 3 months 0.32 – 0.65 mmol/L Repeat in 1 month < 0.32 mmol/L < normal levels Treat immediately Referral to nephrologist Repeat and if < normal levels 25-OH Vit D < 50: deficiency < 75: insufficiency Vit D Rx > 75 Normal PTH assay Abnorma l Referral to nephrologist or internist Normal Urinary fractional excretion of phosphorus if available (if > 20% or > 10% and hypophosphatemia: referral to a specialist Albumincorrected Ca Abnorma l Referral to nephrologist or internist Normal Serum phosphorus 0.65 – normal level Repeat in 3 months 0.32 – 0.65 mmol/L Repeat in 1 month < 0.32 mmol/L Treat immediately Referral to nephrologist < normal levels Repeat and if < normal levels Urinary fractional excretion of phosphorus if available (if > 20% or > 10% and hypophosphatemia: referral to a specialist Serum phosphorus 0.65 – normal level Repeat in 3 months 0.32 – 0.65 mmol/L Repeat in 1 month < 0.32 mmol/L < normal levels Treat immediately Referral to nephrologist Repeat and if < normal levels 25-OH Vit D < 50: deficiency < 75: insufficiency Vit D Rx > 75 Normal PTH assay Abnorma l Referral to nephrologist or internist Normal Urinary fractional excretion of phosphorus if available (if > 20% or > 10% and hypophosphatemia: referral to a specialist Albumincorrected Ca Abnorma l Referral to nephrologist or internist Normal Serum phosphorus < normal levels Repeat and if < normal levels 25-OH Vit D < 50: deficiency < 75: insufficiency Vit D Rx > 75 Normal PTH assay Abnorma l Referral to nephrologist or internist Normal Urinary fractional excretion of phosphorus if available (if > 20% or > 10% and hypophosphatemia: referral to a specialist Albumincorrected Ca Abnorma l Referral to nephrologist or internist Normal Algorithm Algorithm Algorithm Algorithm Algorithm Case Study Aging Woman with longstanding HIV and multiple comorbidities Dr. Gord Arbess Background Information • 62 year old woman • From Jamaica • HIV + since 1996, heterosexual transmission • Nadir CD4 108, VL > 500,000 • Intermittent adherence • Multiple ARV Regimens due to intolerance/resistance (AZT, 3TC, ddI, d4T, Nelfinavir, Amprenavir, LPV, EFV, Indinavir, Tenofovir, RTV) • Hx ABC/3TC HSR Multiple Co-Morbidities • Obese • Hypertension • NIDDM (Gastroparesis-intermittent vomiting) • Sleep Apnea-CPAP • Angina? • Severe Osteoarthritis Knees • Hypothyroid • Hyperlipidemia • Major Depression HIV Medications Present HIV Regimen started June 2012 • Darunavir 800 mg/d • Ritonavir 100 mg/d • Raltegravir 400 mg bid • Etravirine 400 mg/d Other Medications • Lisinopril • Atorvastatin • Ibuprofen • Metformin • Cipralex • Zofran • Eltroxin Routine Bloodwork You notice Serum Cr is 158 (eGFR 48) on routine BW in August 2012 What Would You Do? GFR using CKD-EPI or MDRD < 60 cc/min* ACR and MAU Refer to proteinuria algorithm (next page) < 30 cc/min* CaPO4 Renal ultrasound Referral to nephrologist or internist * If GFR < 50 cc/min: consider adjusting the dose of certain ARV and concomitant medications ** Test for tubulopathy if GFR declines > 10 cc/min while on tenofovir Algorithm Investigations to assess Renal Function • Urinalysis • ACR • Serum Cr (eGFR) • Electrolytes, Bicarb, albumin • Urine for Protein, Cr • Renal Ultrasound • Other? • Biopsy? Results • • • • • • • • • • VL < 40 CD 4 843 Hgb 108 BS 7.3 Hga1c 0.061 ACR 1.1 Trace Protein, no blood, no glucose, 10-15 White cells/hpf, occ red cells/hpf, hyaline casts with some cells Spot urine 0.1 g/L protein, 7.8 mmol/L Cr Cr 118-160 range (eGFR 48-54 range) over number of years Normal electrolytes, normal albumin, normal Bicarb Normal renal Ultrasound (small-sized kidneys) What Would You Do? Urinalysis or urine dipstick Glucose > 0 Protein ≥ 1 + or 0.25 g/L Fasting glucose + Rule out diabetes Repeat at next appt. Glycosuri a DB + Glycosuri a DB – Protein ≥ 1+ or 0.25 g/L Protein < 1+ or 0.25 g/L DB follow-up Repeat 1x ACR and MAU Normal Glycosuri a DB – Referral to nephrologist or internist ACR > 0.05 g/mmol or MAU > 2.1 mg/mmol or hematuria (> 2 RBC/HPF) - Renal ultrasound - Ascertain the risk factors - Referral to nephrologist or internist, or to urologist for isolated hematuria ACR ≤ 0.05 g/mmol and MAU < 2.1 mg/mmol Normal Algorithm What do you think could be accounting for Cr elevation? Etiology • HIVAN? • IgA Nephropathy? • Medication-related? • Hypertension? • NIDDM? • Pre-renal component/volume contraction? • Other? How would you manage this patient? Management Options? • Do you d/c metformin? • Do you d/c NSAIDs? • Do you d/c statin? • Do you Need to dose Adjust ARVs? • Should you Change ARVs? • Do you Hold Ace Inhibitor? • Do you ensure BP/BS well controlled? • Do Nothing? Follow Up • BP well controlled • Hga1c 0.062, therefore Metformin stopped • Asked not to take any NSAIDS • ARV regimen continued at same doses • Continued same dose of statin, ACEi • Cr monitored closely in range of 118-130 (eGFR 55-60 range)